1,2,3-Trimethylimidazolium Iodide: Unlocking New Possibilities in Chemistry
Historical Development
Stepping back a couple of decades, scientists explored ionic liquids for their special properties, searching for new electrolytes and green solvents. 1,2,3-Trimethylimidazolium iodide didn’t just appear out of nowhere; it formed part of this growing interest, following studies into imidazolium salts, which stretched back as early as the 1990s. Early patents and journal entries prove how researchers aimed to harness unique properties by tweaking substituents on imidazole rings. By the 2000s, labs across the US, Europe, and Asia brought this specific iodide onto the radar, thanks to improved synthesis routes and rising interest in materials for solar cells and electrochemical applications.
Product Overview
1,2,3-Trimethylimidazolium iodide usually comes as a white to off-white crystalline powder, packaged in high-density polyethylene bottles for research or industrial use. The appeal of this compound mainly lies in its ionic character and its ability to form stable, conductive solutions. Its strong ionic nature gives it the edge in technologies that count on efficient charge transfer. Labs working with perovskite solar cells, or designers building advanced batteries, appreciate its stable, predictable behavior across a wide range of temperatures and pressures.
Physical & Chemical Properties
In its pure form, 1,2,3-Trimethylimidazolium iodide stands out thanks to good thermal stability, with a melting point typically around 200–210°C. This feature allows scientists to push systems into higher temperature regimes without risk of decomposition. Its water solubility sits toward the upper end for ionic liquids, dissolving readily in polar solvents but less so in nonpolar arrangements. The compound presents as odorless, with a molecular weight hovering just above 250 g/mol. The presence of three methyl groups packs extra hydrophobicity around the imidazolium core, altering both viscosity and solubility compared to its unsubstituted cousins. This helps chemists tailor reactions and application conditions, delivering the mechanical and electrical properties needed for high-value projects.
Technical Specifications & Labeling
Product sheets report purity reaching 98% or better on high-quality commercial products, measured by techniques like HPLC or NMR. Labeling guides show hazard statements referencing potential irritation and environmental concerns. Storage instructions recommend cool, dry, air-tight containers, away from acid fumes and direct sunlight. Clear batch records and analysis certificates demonstrate compliance with ISO standards and traceability, supporting any scale-up from bench to pilot plant. The labeling follows international standards, using GHS pictograms and detailed first-aid recommendations, ensuring anyone handling the material knows exactly what to expect.
Preparation Method
The common laboratory approach starts with 1,2-dimethylimidazole and methyl iodide. Researchers mix these under nitrogen or argon to block unwanted oxidation, carrying out the reaction in anhydrous solvents like acetonitrile. Methyl iodide adds the third methyl group directly onto the imidazole ring, and product crystallizes upon solvent evaporation. Purification runs through recrystallization or, in larger setups, column chromatography. I remember handling reactions like this: careful measurement and slow addition of methyl iodide prevent byproduct formation and help workers steer clear of over-alkylation or side reactions. Every successful batch comes down to tight control over reaction temperatures and rigorous exclusion of moisture.
Chemical Reactions & Modifications
This iodide salt finds itself at the center of many creative modifications. In academic labs, chemists rely on nucleophilic substitutions, where the iodide can exchange for other halides or functional groups, granting access to entirely new imidazolium derivatives. The methyl groups restrict the reactivity on the ring’s backbone, giving a cleaner, more directed transformation. The cation acts as a carrier for various reactions—making it valuable not just in synthetic chemistry but also in engineering advanced functional materials. Electron transfer reactions, halide metathesis, or even ionic liquid formation all benefit from its structural rigidity. These pathways open the door to new classes of electrolytes, membranes, and sensor systems.
Synonyms & Product Names
1,2,3-Trimethylimidazolium iodide has cropped up under several synonyms throughout scientific literature. You’ll spot it labeled as TMI-I, N,N',N''-Trimethylimidazolium iodide, or 1,2,3-Trimethyl-imidazolium iodide on reagent bottles. Different catalog numbers reflect supplier preferences—a reminder to double-check chemical structure or CAS number before ordering. These alternate names often turn up in older patents or international publications, but chemists always return to the core structure: three methyls and a single iodide counterion.
Safety & Operational Standards
Safety ranks top of mind, especially because methyl iodide brings its own hazards to the synthesis. Proper handling includes gloves, chemical-resistant goggles, and local exhaust ventilation. Iodide salts sometimes act as mild irritants for skin and eyes, and their dust can bother airways. Safety data sheets highlight emergency measures, from eyewash stations to containment of spills. Waste goes straight into halogenated organics bins or specially marked containers, following city or state regulations. Lab teams meticulously label secondary containers and communicate hazards during hand-offs, keeping everyone in the loop and risks minimized.
Application Area
Solar energy research fueled interest in this compound over the last decade, especially in perovskite and dye-sensitized solar cell prototypes. Here, 1,2,3-Trimethylimidazolium iodide enables stable, high-conductivity electrolytes that boost both efficiency and device longevity. It plays a role in electrochemical sensors, acting as part of signal-amplifying ionic liquids. Studies on corrosion inhibition and new battery types increasingly include this salt, thanks to its high electrochemical window and stable ion transfer. Pharmaceutical research also touches on these derivatives, not as active ingredients, but for crystallization or extraction of target molecules.
Research & Development
Global labs keep pushing boundaries using this compound. Teams compare its electrolyte performance with other imidazolium salts, searching for a balance of power output and operational stability. Research alliances in Europe have tested it in solid-state batteries, tracking improvements in conduction and temperature tolerance. In Asia, material scientists use it to tune the interface between active layers in solar devices, looking to cut cost and energy losses. Collaborations with chemical engineers sharpen manufacturing processes—switching up solvents to cut environmental impact and examining recycling streams for spent ionic liquids. These partnerships bring fresh eyes and new approaches, driving innovation forward.
Toxicity Research
Early on, imidazolium iodides looked promising for green chemistry, but researchers soon spotted some challenges. Animal studies and in vitro tests confirm low acute toxicity, though chronic effects and long-term exposure risks stay under review. Environmental persistence remains a hot topic. Iodide ions pose minimal threat in most settings, but methylated imidazolium can undergo slow biodegradation, especially in aquatic environments. Regulatory agencies in Europe and North America issued guidelines on discharge concentrations, drawing on a handful of toxicity studies. Research continues, with labs developing advanced life cycle assessments and safer analogues. This attention gives industry a way to weigh benefits against impacts, moving toward smarter chemical stewardship.
Future Prospects
People want safer, more powerful energy storage and generation technologies, so demand for compounds like 1,2,3-Trimethylimidazolium iodide will probably only climb. Ongoing tweaks to its chemistry could bring even better results, like wider voltage stability or faster switching times for next-gen electronics. The path involves tailoring both the cation and anion, possibly blending with organic or polymer matrices for new hybrid materials. With global teams scrutinizing both environmental effects and industrial performance, the outlook for this molecule stays bright—offering tools to tackle urgent problems in clean energy, electronics, and analytical chemistry. The next decade could see this compound anchoring even more discoveries across scientific fields.
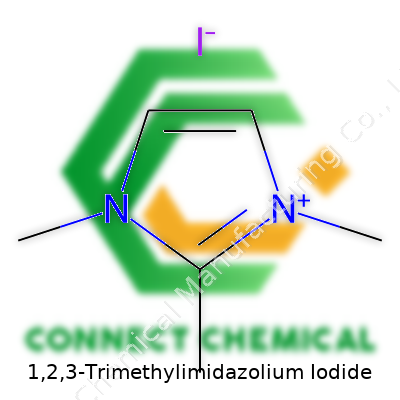
A Clearer Look at the Structure
1,2,3-Trimethylimidazolium iodide is a mouthful, but breaking it down helps. At its core, there's an imidazole ring, a five-membered ring made from three carbon atoms and two nitrogen atoms. When you see the name, “1,2,3-trimethyl” points to three methyl groups — simple chains of one carbon and three hydrogens — attached at the first, second, and third positions of the ring. Each methyl group nudges basic hydrogen atoms out of their usual spots, changing both shape and chemical behavior.
The next piece, iodide, works as a counterion here. The imidazolium chunk holds a positive charge since these methyl groups push out more than just hydrogens — they can affect electron sharing inside the ring. This extra charge looks for balance, so the negative iodide ion pairs as a close companion. The finished look? A cationic (positively charged) imidazolium surrounded by a big, chunky iodide ion.
Why Structure Matters
Seeing the actual structure opens up the reasons behind its importance. Try sketching the imidazole ring, then pop methyl groups at 1, 2, and 3, and you’ll notice how this set-up tweaks the molecule's symmetry and charge distribution. Thinking back to basic organic chemistry, three methyls stuffed side-by-side make the ring more rigid, less likely to twist or interact with neighboring molecules in the ways you’d expect from a plain imidazole.
Chemical properties shift, too. These changes influence melting points, solubility, and how the molecule dissolves or reacts in different solutions. Swap the iodide for something else, and suddenly, its behavioral patterns change again. Synthetic chemists, in pharmaceutical labs or developing energy storage, watch these details closely because they tweak performance and reactivity. In fact, ionic liquids built from imidazolium cations — just like this one — have turned up everywhere, from battery electrolytes to supercapacitors, even acting as solvents in green chemistry experiments.
Context and Real-World Value
I’ve seen research teams strike gold after swapping out a single methyl group. That’s the scale of effect we’re talking about: changing three hydrogens to methyl groups in such a small scaffold makes a huge difference. The tight pairing with iodide adds extra stability in some applications. For example, electrolytes containing this compound can survive voltage swings that break down more traditional salts. Some papers point to its stability and compatibility with perovskite solar cells, pushing up efficiencies thanks to these subtle chemical tweaks.
One issue with compounds containing iodide involves storage and handling—exposure to air or light sometimes leads to side reactions that form iodine, which troubles researchers working with sensitive equipment. This challenge is nothing new in chemical labs. Good packaging and storing under inert gas, or in dark glass, keep the material ready for high-end applications. The structure itself encourages creativity in solving these bottlenecks, pushing researchers toward new stabilizing additives or packaging solutions.
Room for Growth and Innovation
Getting more from 1,2,3-Trimethylimidazolium iodide means keeping an eye on chemical structure and its impact. Every tweak opens up new opportunities for design. Developing sturdier imidazolium-based solutions for batteries, solar cells, or synthetic biology will demand even more keen focus on structural detail. In the end, it’s the interplay between these small chemical changes and the big advances they unlock in real technology that continues to drive new research — and future breakthroughs depend on understanding those bonds, charges, and ring positions as well as the next set of curious eyes.
Role in Modern Research and Chemistry Labs
Anyone who’s spent time in a university chemistry lab or a materials science department starts to notice certain names bubbling up in project meetings and supply closets. 1,2,3-Trimethylimidazolium Iodide pops up regularly, especially in places focused on electrochemistry and advanced materials. It’s a salt, but not the kind you shake on fries—it pulls double duty as a building block and facilitator in some cutting-edge projects.
Solar Cells: Powering the Next Step
Most folks don’t look at a rooftop solar panel and wonder about the chemistry inside, but if you did some digging, you’d find compounds like 1,2,3-Trimethylimidazolium Iodide making a difference. Perovskite solar cells, in particular, grab headlines for their promise and the creative chemicals involved. This salt helps improve the stability of the active layer and boosts how efficiently cells can convert sunlight to electricity. Research teams use it as an additive or precursor, fine-tuning results by tweaking how much they introduce or how they combine it with other components.
A report in the Journal of Materials Chemistry laid out that swapping certain iodides and tweaking molecular structure made cells more durable. Based on what I’ve seen in labs and read in peer-reviewed studies, adding this salt often means fewer failures in moisture-prone conditions—helpful when trying to scale solar panels for outdoor use.
Key Ingredient in Redox Chemistry and Batteries
Talk to anyone building experimental batteries, and they’ll mention ionic liquids: salts that melt down into liquid form close to room temperature. 1,2,3-Trimethylimidazolium Iodide fits right into that family, lending itself as an ionic liquid or being dissolved in existing blends. That means better conductivity, plus more flexible choices when picking solvents and materials for batteries or even sensors.
Redox flow batteries and dye-sensitized designs count on it not just for performance, but also for safety—keeping thermal runaway risks low and improving the way electrons zip around inside the cell. Scientific papers from the American Chemical Society mention how changing the imidazolium group's structure alters conductivity, letting researchers target specific properties for more robust performance.
A Tool in Organic Synthesis
Making pharmaceuticals, dyes, and other fine chemicals often calls for a clever hand in assembling molecules. Salts like 1,2,3-Trimethylimidazolium Iodide act as catalysts or phase-transfer agents, coaxing chemical reactions that otherwise stall out. A graduate student I worked with found yields jumped significantly using this salt as a supporting electrolyte, cutting waste while hitting target molecules more efficiently.
Companies chasing quicker, safer, and greener chemical synthesis lean on these types of salts to cut down on harsh reagents. Journals such as Advanced Synthesis & Catalysis cite lower reaction temperatures and improved selectivity, which links directly to safer workplaces and fewer hazardous byproducts.
The Takeaway for Scientists and Engineers
Sometimes obsessing over molecules and their quirky forms pays off not just for researchers in a lab, but for anyone flipping a light switch. 1,2,3-Trimethylimidazolium Iodide only hits the news when a solar efficiency record breaks or a new battery design shows up at a conference. Still, from what scientists and journals report, this salt captures a rare mix of reliability and versatility. Growing interest around safer solvents, efficient power storage, and cleaner manufacturing means this particular imidazolium salt is likely to show up in more applications and more headlines.
Understanding Its Quirks
Not every chemical ends up sitting quietly in a storage room. 1,2,3-Trimethylimidazolium Iodide stands out for its hygroscopic nature—meaning it enjoys sucking up moisture from the air. Left out long enough, it clumps or dissolves, even if you started with a clean, dry powder. This property changes how it behaves in reactions and messes with measurements, turning routine lab work into a guessing game. Getting the facts straight on storage and handling helps keep mistakes out of the process.
Storing It Right
Dryness remains enemy number one for this salt. Screw-cap bottles with tight seals and a label showing the date and lot let you manage exposure. Workers I’ve met in research settings prefer to stash it in a desiccator—throwing in a fresh silica gel pack, too. Even at room temperature, a closed desiccator box helps the chemical stay stable. Iodide salts also respond poorly to light; translucent containers or open benches don’t cut it. Using amber glass limits UV exposure and stops light-induced changes that could leave the compound partly degraded or colored.
Beyond dryness and darkness, clearance from acids or strong bases matters. The iodide ion, the heart of this compound, reacts with oxidizers—sometimes with surprising vigor or toxic byproducts. Keeping storage shelves organized, with separate bins for reactive chemicals, goes a long way. This avoids those moments where a cracked cap or a splash turns routine inventory into a hazardous waste call.
Safer Handling: More Than Labels
Preparation before weighing this dust-like substance pays off. It wafts easily, so even a light breeze or careless pouring spreads it thin across the bench. In practice, users work over dedicated spill trays and clean up immediately. Gloves and goggles aren’t just for show—the fine particles can spill onto hands or stir up, and iodide compounds sometimes stain skin or irritate mucous membranes.
Iodides feature another wrinkle: over time or in warm conditions, the material can pick up a slight iodine smell, hinting at slow breakdown. A one-way ticket to accidental exposure comes from ignoring this sign. Regular visual inspections catch problems before they escalate, as does weighing only what you plan to use, then closing the container fast. Even after decades in labs, small habits like writing the opening date and periodic checks make the difference in both research consistency and on-the-job safety.
Disposal Done Right
Contaminated glassware or leftover powder won’t just rinse away down the drain. Local regulations treat iodide salts with care, mostly due to their potential environmental and health impacts. Neutralizing the substance, followed by collection in a sealed waste bucket labeled as hazardous, protects both staff and the surrounding ecosystem. On big campuses or industrial sites, teams work closely with hazardous waste vendors, making sure every gram leaves the building safely.
Real-World Solutions
Investing in clear storage protocols and routine safety training saves more than just time—it cuts down on exposure risk and reduces waste from spoiled material. Regular inventory checks, dual-signature protocols for opening rare chemicals, and batch-tracking simplify compliance and keep costly reagents from expiring unnoticed. In the end, building these habits isn’t about following a checklist. It’s about respecting the quirks of compounds like 1,2,3-Trimethylimidazolium Iodide so that science—and scientists—stay out of trouble.
Chemical Exposure Risks in Everyday Labs
Plenty of chemicals used in labs don’t exactly fall under the category of household names, and 1,2,3-Trimethylimidazolium iodide fits that bill. Chemists and materials scientists poke at it looking for electric or catalytic performance. At the bench, safety concerns pop up quickly even before the first reaction starts.
Any time someone handles this compound, skin and eye hazards stay on the radar. Even brief contact with fine dust, solution, or residue creates opportunities for irritation or allergic responses. Laboratories I’ve worked in rely on nitrile gloves, splash goggles, and lab coats to keep the skin and eyes covered because stories of chemical burns circulate easily.
Breathing in Trouble
Airborne dust poses its own set of problems. Breathing in just a bit of a compound like this—especially when weighing or transferring—throws the door open for respiratory irritation and headaches. A few minutes without a fume hood can create a situation where a simple cough or sore throat lingers all day. Education on good airflow, using powder carefully, and always working in ventilated spaces can cut down on these accidents by a big margin.
These are lessons learned from stubborn coworkers who insisted on short cuts. Getting caught up trying to clean up accidental spills with just a damp towel only made matters worse. Every year, lab safety reports across universities and chemical companies end up showing how a loose attitude around respiratory hazards costs time, money, and sometimes a hospital visit.
Potential for Chemical Reactions
Reactivity matters, too. 1,2,3-Trimethylimidazolium iodide sits at the crossroads for some aggressive chemistry. Mixing with strong oxidizers or other incompatible reagents brings a risk of toxic gas release or even fire. Open bottles, forgotten reactions, or poor labeling usually lead to emergency alarms, not scientific breakthroughs. The number of “near miss” events I’ve witnessed come down to overlooked details during a rush.
Most chemical inventories end up with a list of incompatible substances. Still, pressure to cut corners sometimes outweighs the warnings. Forgotten bottles or unmarked beakers quickly turn into headaches during lab clean-ups and inspections. People who keep track of all chemicals and their risks improve safety for everyone, even long after the original project wraps up.
Environmental and Chronic Health Questions
Disposal practices stand out as another area with real stakes. Dumping this chemical into sinks, or mixing with regular trash pick-up, brings downstream problems. Environmental impact assessments show that improper disposal routes let these chemicals leach into wastewater and soil, causing ripple effects nobody intended.
Workers exposed to repeated low doses deserve attention, too. Studies of similar ionic liquid compounds indicate potential long-term harm to internal organs or developing fetuses, especially when employees skip gloves and ventilation out of habit or perceived inconvenience. Health monitoring, reporting even minor symptoms, and regular training can catch issues before they grow.
Practical Steps Toward Safer Labs
Several approaches make a difference in real labs. Clear labeling, good housekeeping, and up-to-date Safety Data Sheets help keep everyone on the same page. Technicians, students, and postdocs who treat every tiny spill or questionable bottle as a genuine hazard build a resilient culture. Investment in personal protective equipment, regular drills, honest conversations about incidents, and clear planning for waste disposal can close most safety gaps.
Understanding Purity in the Lab
Anyone who’s mixed chemicals or chased reactions down to the decimal point knows that purity makes or breaks the outcome. This isn’t just a lab myth. With 1,2,3-Trimethylimidazolium Iodide, most researchers seek out materials hitting at least 98% purity. Below that, byproducts or contaminants start interfering. It’s frustrating to troubleshoot bad data only to discover the cause traces back to impurities in a single reagent.
Ultra-high purity—up to 99% and above—often draws in those chasing precision for high-tech or pharmaceutical work. You pay for that extra percent, but I’ve seen what a contaminant at just half a percent can do to a synthesis yield or NMR result. On the other hand, working with slightly lower purity sometimes works for early feasibility tests or when the chemical goes into a non-sensitive step in a process.
Real-World Packaging Sizes
I remember my graduate days, pooling grant money and scraping out every milligram from tiny glass bottles. Chemicals like 1,2,3-Trimethylimidazolium Iodide commonly arrive in 1 gram or 5 gram vials for good reason. These sizes fit bench-scale experiments and keep costs lower for those looking to run a handful of reactions. Packaging in these small sizes also cuts down on waste, reduces risk of accidental exposure, and means less substance to store or dispose of.
Custom orders and larger quantities—for example, 25 grams, 100 grams, or even kilo-scale formats—serve companies scaling up reactions. By the time you need a bottle bigger than your coffee mug, you’ve probably cleared regulatory hurdles and repeated the process dozens of times. Larger labs, teaching institutions, and R&D departments usually keep at least a 25-gram stock on hand unless monthly demand points higher.
Some specialty suppliers offer even more flexible sizing. You can request custom aliquots or packaging with additional safety features, like light-proof containers to shield from degradation or resealable pouches for moisture-sensitive work. I once asked a supplier to split a 50-gram order into five separate containers after a mishap taught me how easily moisture can ruin an entire batch.
Purity and Size Tied to Application
Where the chemical heads next really sets the choice of grade and packaging. University research groups often stick with 98–99% grades in 1 to 5 gram sizes. Analytical houses may ask for certification of analysis, running their own checks before trusting a new lot. Industrial clients, especially those moving to pilot scale or production, tend to deal with 25 grams and above and sometimes push for purification beyond standard catalog listings.
Direct conversations with supplying chemists make a difference. Reliable companies always list purity clearly, link to batch-specific data, and offer technical support if your process calls for something a little outside the norm. If a supplier seems hazy about quality control, that’s a red flag I’ve learned not to ignore. With high-purity chemicals, trace metals, water content, and even packaging environment can all leave fingerprints on the end result.
Moving Toward Transparent Supply Chains
Broader trends push toward certified supply chains and digital tracking. Researchers and quality managers ask not just about what’s inside the bottle but also where it was made, who handled it, and how long it sat on a shelf. Big suppliers lead here, but independent labs increasingly request this info to back up published work or regulatory filings.
Overall, picking the right purity and pack size of 1,2,3-Trimethylimidazolium Iodide isn’t just a box-checking exercise. It reflects trust in your experiments, the safety of your team, and the data or products you produce. Ask questions, look for the details, and don’t accept vague answers when your results, or reputation, hang in the balance.