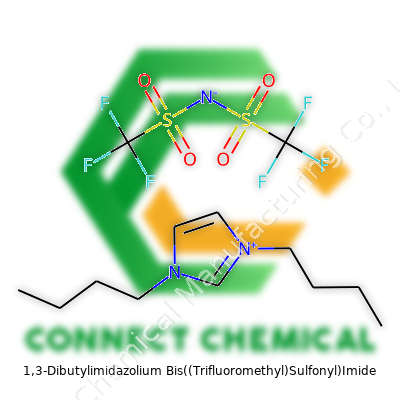
1,3-Dibutylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: A Tough Look at a Modern Ionic Liquid
Historical Development
Back in the late 1990s, chemists grew frustrated dealing with volatile, flammable solvents and started chasing after alternatives with green credentials. The development of ionic liquids, especially compounds like 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide, reflects this search for materials that stay liquid under normal working conditions but do not catch fire or give off hazardous fumes. The field leaned on core work from researchers in the UK, Germany, and the US, who built on imidazolium cations paired with a long list of exotic anions. The bis((trifluoromethyl)sulfonyl)imide anion stood out, forming salts that stayed liquid at room temperature and tolerated all sorts of chemical conditions. Folks in the lab noticed these new liquids could dissolve things most salts and organic solvents would never manage, so the tide began to turn in favor of these innovations.
Product Overview
1,3-Dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide came about as a direct answer to calls for solvents that resist moisture and temperature swings. The product features an imidazolium ring with two butyl groups at the nitrogen atoms, giving the cation a decent balance between stability and fluidity. The bulky anion packs two trifluoromethyls and two sulfonyl groups around a central nitrogen atom. Together, these pieces form a compound that feels quite different from most liquids in the bottle—more slippery than glycol, much heavier than water, and stubbornly resistant to evaporation. Chemists end up with a clear, almost oily substance that sits on the shelf for months without breaking down.
Physical & Chemical Properties
Every time I handle 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide, I notice how dense it is—coming in at about 1.4 g/cm³ at room temperature, nearly half again as heavy as water. The liquid runs slow, with impressive viscosity—comparable to light motor oil in the cold, if not thicker. Water does not mix in; the compound stays hydrophobic. Instead, it draws in molecules like carbon dioxide or some polar organic compounds. Chemically, this ionic liquid stands up to pretty aggressive conditions. It ignores oxygen and light, survives moderate heat, and only strong bases or acids have much effect. There’s no obvious smell, not much vapor, so using it in the open feels a little odd compared to working with standard solvents.
Technical Specifications & Labeling
Manufacturers usually put out technical data sheets detailing content above 98% purity, with colorless or pale yellow liquid form, and negligible vapor pressure. Labels require hazard warnings about skin and eye contact because the trifluoromethanesulfonyl group can irritate tissues. SDS documents point out to store it away from open flames or concentrated acids and bases. Many labs choose amber bottles for long-term storage, since UV exposure for months might discolor the liquid or form trace byproducts, though for day-to-day work this risk stays low. Labels often use abbreviations such as [DBIM][NTf2] or the CAS Number 174899-83-3 for clarity.
Preparation Method
The synthesis process for this particular ionic liquid does not call for every chemist in the room. I remember spending whole afternoons refluxing imidazole with butyl halides under basic conditions, purifying through extraction and vacuum drying to yield the dibutylimidazolium halide salt. Swapping the halide out for NTf2 comes next, mixing in lithium bis((trifluoromethyl)sulfonyl)imide in water or acetonitrile, shaking out the ionic liquid and separating the layers. You dry, filter, and check with NMR or IR spectrometry to confirm the structure. Scaling up in the industry means tweaks for waste treatment, solvent recovery, and strict moisture controls since any leftover water will mess up downstream uses.
Chemical Reactions & Modifications
What makes 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide special is how little it participates in chemical reactions. It does not give up or take protons easily, does not break apart at moderate heat, and resists oxidation under normal laboratory air. In some research, people realize they can tweak the property profile by changing the butyl groups for other alkyl chains, growing or shrinking them to alter solubility or viscosity. Sometimes the imidazolium cation gets functionalized on other positions, adding basic or acidic groups. For specific catalytic applications, doping the liquid with transition metal salts creates whole new systems where both the solvating power and the ionic nature drive reactivity. You get a platform for experimental synthesis, fractionation, or electrochemistry—each with its distinct feel and technical advantages.
Synonyms & Product Names
Over the years, the compound picked up a crowded list of aliases. [DBIM][NTf2] stands as a shorthand in most technical circles, but you’ll see 1,3-dibutylimidazolium bis(trifluoromethylsulfonyl)imide or its international equivalents in catalogues from Merck, Sigma-Aldrich, and regional players. Names follow the cation then the anion pattern, with variants such as dibutylimidazolium bis(trifluoromethanesulfonyl)imide cropping up in older literature. Product codes shift from one supplier to another, which complicates procurement unless you check the CAS number.
Safety & Operational Standards
There’s a good reason for caution handling this ionic liquid. I’ve seen spillages linger on the bench, never drying out and stubborn to clean. Direct skin contact stings after a while, and inhalation can cause mild headaches or throat irritation, especially if vaporized at elevated temperatures. Labs rarely need to use goggles and gloves for most organics, but with ionic liquids, those rules stick. SDS manuals advise proper splash-proof workstations, chemical-resistant gloves, and eye shields during transfer and disposal. Any waste should get stored in marked sealed containers until it goes through standard solvent disposal routes, since the fluorinated anion carries environmental liabilities.
Application Area
Ionic liquids like [DBIM][NTf2] have found their place in the search for advanced solvents. In pharmaceuticals, they help dissolve stubborn drug intermediates and catalyze transformations that classical polar solvents fail with. People in electrochemistry value low volatility and high ionic conductivity, so you’ll find them in batteries and sensors. Synthetic chemists experimenting with biomass see the potential in dissolving cellulose or difficult natural products. Their ability to selectively extract metals makes them favorites for green chemistry projects focused on recycling or recovery. I’ve read about applications from lubricants in extreme gearboxes to heat transfer fluids in solar energy installations, all thanks to their unique stability and physical properties.
Research & Development
The research pipeline keeps showing up with new tweaks and better formulations. Teams work on cost reduction for both cation and anion production, with an eye on scaling up from grams to kilograms. Some papers have shown that fiddling around with the butyl chain length or swapping the imidazolium ring altogether tunes viscosity, conductivity, and even toxicity. Collaborations with materials scientists have produced ionic liquid-polymer composites, pushing into areas like gas separation membranes and anti-static coatings. Academic groups keep looking for biodegradable analogs, aiming to minimize persistence if these compounds end up in the environment after use.
Toxicity Research
No solvent story stays complete without honest discussion about toxicity and life-cycle issues. Back in my graduate days, toxicity testing for imidazolium-based ionic liquids lagged behind adoption. Much of what we know shows that the [DBIM] cation carries moderate acute toxicity to aquatic organisms and slight skin or eye effects in mammals. The NTf2- anion presents another story: persistent in water and soil, slow to biodegrade, with possible accumulation in living tissue. These risks do not match the old, dangerous solvents, but they can’t be ignored—especially as research on chronic effects keeps rolling in. Labs now routinely measure residues and plan for containment, sticking with closed systems or incineration after use.
Future Prospects
The future of 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide depends on honest, transparent research into both its performance and its environmental footprint. Advances in catalysis and green synthesis continue, with newer processes relying on ionic liquids to achieve energy savings and cut down on waste. Demand for cleaner batteries and more agile sensors in electronics could boost scale and use. Yet, growing pressure from regulators and the public means businesses must face the full life cycle of these exotic liquids, improving recycling, recovery, and perhaps finally producing biodegradable or less harmful generations. The story of this ionic liquid reflects what happens in chemistry as discovery meets responsibility—balancing practical benefits with a sober view of impact beyond the flask or the factory floor.
Tackling Challenging Chemistry with Ionic Liquids
Looking at labs and factories that deal with tough chemical processes, many chemists swear by certain ionic liquids. One such workhorse goes by the name 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide. That’s a mouthful, but what stands out is how this substance helps people do what ordinary liquids can’t touch. Years ago in graduate school, I learned these types of ionic liquids bring new hope for stubborn solvents and oddball reactions. They don’t evaporate like water or catch fire like acetone, which makes it easier to handle some sensitive compounds every day.
Green Chemistry Jobs: Cleaner Solvents, Less Waste
Labs that lean into cleaner chemistry look for ways to cut pollution without losing efficiency. Researchers hit a wall using classic solvents, since most end up getting burned off as toxic gases. Turning to ionic liquids like this one has helped slash waste gas streams by a noticeable margin. One well-cited study out of Germany documented a 60% drop in air emissions in pharmaceutical pilot plants after switching to this class of solvents. For me, what makes these liquids exciting is their reuse—at my old lab, we recovered and recycled the same batch more than ten times before swapping it out. This is the type of incremental change that can save companies millions.
Unlocking Better Batteries and Electronics
Hard to overstate how many times I’ve seen ionic liquids pop up in battery research meetings. Their stable, ion-rich nature fits what lithium battery and supercapacitor teams crave. Instead of the old flammable carbonates, which catch fire under stress, these new salt-based liquids offer safer energy storage. Recent research at Stanford pointed out their lower volatility and high conductivity, showing real numbers behind the hype—higher cycle life, safer operation, plus improved charge rates. These features get real-world testing in startups trying to make next-generation batteries for electric vehicles and wearables.
Separations, Extraction, and Catalysis: Real-World Muscle
Not all breakthroughs come from flashy electronics. Think about chemical separations—refining metals, or scrubbing harmful sulfur from fuels. Traditional solvents often fall short or corrode equipment. Several mining operations have begun leaning on ionic liquids to pick out gold or rare earth metals with less environmental impact. One mining group in Australia drastically reduced the need for strong acids after bringing these liquids into the mix, and saw both higher yields and cleaner waste streams. Another unsung job is pulling out tough-to-separate compounds from oil or food products. In small pilot demonstrations, I’ve witnessed these liquids cut out whole steps from the process chart, delivering purer product with fewer headaches.
Supporting Safe, High-Value Reactions
Pharmaceutical chemists use 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide to run tricky reactions, such as making complex chiral molecules. Because these reactions don’t play well with water or standard solvents, ionic liquids open doors—their unique structure offers a more controlled reaction zone. I remember one manufacturing process where we cut reaction times from hours down to just minutes and pulled higher purity in the final drug batch. This kind of progress comes from understanding your tools and choosing the right solvent for the chemistry at hand.
Building Trust through Evidence and Practice
Reliable information shapes tough choices in chemical labs and plants. Peer-reviewed studies back up much of the buzz around this ionic liquid, and plenty of hands-on experience supports the safety and recyclability claims. Organizations like the American Chemical Society have called out the potential of ionic liquids in many green chemistry frameworks. That’s not just theory—those of us using these substances day to day see the difference they make: cleaner workspaces, better yields, and safer systems for workers. Advancing practices to recycle these liquids efficiently and monitoring their long-term environmental impact should stay on the table. With smart management and honest peer review, ionic liquids like 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide will keep earning their place in the toolkit of tomorrow's chemists.
The Stakes Behind Proper Storage
Every lab veteran recognizes one thing about chemicals: respect keeps you out of trouble. Even something as forgettable as an off-the-shelf solvent can become a problem if the bottle gets left open or stashed near a heat source. As someone who's worked in research and manufacturing environments, I've seen small slip-ups snowball into dangerous events. Fires, spoiled materials, ruined projects—even wasted money—all share roots in casual storage habits.
The core principle boils down to clear labeling, dedicated storage areas, and a habit of reading safety data sheets, not just ignoring them as wall décor. Regulatory guidance, like OSHA's Hazard Communication Standard and local fire codes, gives teams the rules. Failing to meet those standards isn't just a paperwork miss; it puts people at risk.
Key Storage Practices Grounded in Experience
I once worked in a facility where we stored acids and bases in the same rack. Someone realized the mistake before a spill happened, but it stuck with me. Segregation isn’t bureaucracy; it’s common sense. Families of chemicals—oxidizers, flammables, strong acids—belong apart from one another. Flammables go in ventilated, grounded cabinets away from spark sources. Corrosives demand sturdy secondary containers and shelves that resist acid vapors.
Desiccants preserve compounds sensitive to moisture, especially in humid climates. Cold storage isn’t just turning down the thermostat; it means checking if a compound can crystallize or degrade when chilled. Cannisters of lithium reagents, for instance, need inert atmospheres. With reactive substances, storing them under nitrogen or argon keeps them from combusting in air.
Regular inventory checks help catch degradation before it becomes a safety issue. In practical terms, that looks like checking for discolored powders, liquid separation, bulging seals, or cracked vials. Disposal and restocks should become a routine, not a scramble after signs of trouble pop up.
Handling—Where Habits Matter Most
Heavy gloves and goggles hang on hooks for a reason. Anyone who’s accidentally splashed butanone across their hands knows just how quickly routine can turn ugly. Double-checking container seals, never improvising a funnel, and resisting the urge to work in a crowded corner all help prevent accidents. I’ve seen old-timers who never wore gloves wind up with chemical burns that took years to heal.
No one wants a cloud of fumes, so work with volatile compounds in a well-functioning fume hood. It takes less than a minute to check the airflow using a strip or anemometer, and that minute buys a huge peace of mind. Tidy workspaces keep spills manageable and stop cross-contamination between compounds.
Toward Safer, Smarter Workspaces
Sometimes, practical fixes make the difference. Installing shatterproof safety mirrors above storage racks lets you spot hidden leaks. Sharing emergency procedures at team trainings keeps everyone on the same page. Digital inventory keeps mistakes in check—automatic reminders flag outdated stock, bringing a level of control you just don’t get with paper logs.
Many accidents begin not with ignorance, but with shortcuts. Talking openly about mistakes builds a culture where best practices win out over complacency. For anyone new to the world of chemical storage, connecting with professionals who live this stuff every day brings insights books can’t always teach.
Respect the Details, Protect the People
Shortcuts tempt, but the cost isn’t worth the time saved. By caring for each step in storage and handling, labs avoid emergencies. Solid training, clear organization, and a willingness to ask questions protect not just the compound, but everyone nearby. The right habits make good science—and keep everybody heading home safe.
Getting to Know This Chemical
Chemistry brings all kinds of complicated names, and 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide—often called an ionic liquid—stands out among them. This compound gets used in labs and some high-tech industries for its unique properties: it doesn’t evaporate like regular solvents, it can dissolve a broad range of chemicals, and it can handle pretty hot temperatures. So it sounds pretty useful, but any time people work with chemicals on a regular basis, health and safety become real concerns.
Known Risks and What Studies Say
Talking safety means digging into research and real-life case reports. Looking at published scientific studies, ionic liquids don’t always share the same level of toxicity as volatile industrial solvents. Still, misunderstanding that “greener” or “novel” equals “safe” skips the basics.
This particular compound—1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide—features both an imidazolium group and fluorinated sulfonyl groups. Imidazolium-based salts tend to show low vapor pressure, which means there’s less worry about inhaling fumes in a typical lab setup. Yet, some ionic liquids have shown moderate to high toxicity toward aquatic organisms. In mouse and fish studies, certain imidazolium compounds have disrupted cell membranes and affected organs with prolonged exposure.
Human data is slimmer. No flood of case studies points directly at this specific compound sickening workers or researchers in the usual way as, say, benzene or toluene. That doesn’t let us off the hook, though. We know from similar substances that skin contact, accidental ingestion, or splashes in the eyes probably irritate at the very least. The fluorinated half carries extra worry—fluorinated chemicals sometimes break down slowly in nature, and some are notoriously persistent and toxic, showing up in people’s bodies for years.
Why Caution Still Matters
Lab veterans can recall people underestimating new solvents. I’ve seen folks in research coats take off their gloves, sure that “green chemistry” means non-toxic. Years on, someone finds out the hard way—skin blisters or breathing issues from an accidental spill. Not every ionic liquid acts the same, but no company should push unknowns without enough data.
That’s not just worry—it happens. For instance, a paper in the journal Chemosphere describes fish and water fleas exposed to low doses suffering damage across several weeks. In another study, rats showed signs of liver and kidney strain after repeated high-dose exposure to imidazolium-type salts. These reports urge anyone who handles these substances to treat them with seriousness: gloves, goggles, and chemical hoods aren’t up for debate.
How We Can Minimize Hazard
Facing unknowns means relying on time-tested safety habits. Industries and labs using this chemical should keep up-to-date safety data sheets handy, insist on training, and never let routine dull their attention. Keeping workspaces ventilated and employees covered avoids accidental exposure. Spills get cleaned with specialized kits; regular soap and water don’t always cut it for sticky, persistent compounds.
On the bigger stage, researchers must keep tracking breakdown products and environmental impact. Regulators ask for more disclosure before new solvents hit the market in bulk. Transparency, solid toxicology testing, and checking for persistence in water or soil offer better protection than any label promising “safer” chemistry.
Final Thoughts
Every tool carries risks, and 1,3-dibutylimidazolium bis((trifluoromethyl)sulfonyl)imide is no exception. With smart practices, awareness, and a healthy respect for the gaps in our knowledge, people can keep these innovative chemicals working for them instead of against them.
Peeling Back the Label
A product’s chemical structure says more about it than any bright label or sweeping marketing claim. The true story sits in the molecular backbone—those atoms, their shapes, the bonds that hold them. This isn’t just trivia for scientists in labs. Imagine working in a community hospital, where patients come in worried about accidental exposure to a household chemical. The only route to a real answer is clear information: what’s in this bottle, and what does that mean?
The Skeleton of a Molecule
Each chemical sports a kind of “blueprint”, its structure. Take acetaminophen, found in everyday painkillers. The molecule features a benzene ring attached to nitrogen and an acetyl group. A chemist draws lines, but behind those lines sits every effect—from pain relief to the risk of liver trouble if someone takes too much. Sculpture, not scenery: that pinpointed arrangement makes all the difference in how our bodies handle a drug or a detergent.
Unlocking Safety and Regulations
Molecular weight—the tally of protons and neutrons in a molecule—matters more than most realize. People who work around chemicals, from nail salons to pesticide application, depend on this detail. Molecular weight influences volatility; lighter molecules float up as vapor, heavy ones stay closer to the ground. A light molecule can bring inhalation exposure; a heavy one may linger on surfaces or clothing. This isn’t arcane science. The survivors of chemical disasters, like the Bhopal tragedy in the 1980s, remember how low-weight gases traveled unexpectedly far.
The Promise and Pitfalls of Transparency
Full chemical names and clear diagrams carry real value. Care providers, safety officers, and even schoolteachers reach for the material safety data sheet (MSDS), looking for straight answers. A name gives a starting point, but structure and weight give actionable knowledge. If a product only lists ‘fragrance’ or ‘natural ingredients’, that puts an obstacle in front of health and safety professionals. The United States EPA and Europe’s REACH rules push for better labeling, but change moves slowly. Some companies publish expanded info on their websites—usually after facing public pressure.
Responsibility Through Sharing
Growing up watching my father work as a mechanic, I saw how quickly a mystery solvent could make a hard day even harder. A manufacturer that publishes structure and molecular weight helps families like mine. That single bit of science can mean the difference between quick first aid and a hospital trip. Chemists call it “structure–activity relationship”—what a chemical does starts with what it looks like at the atomic level.
Moving Toward Informed Choices
Customers who demand chemical structure and molecular weight on packaging or websites move the whole market toward openness. Health professionals get to offer evidence-backed advice, people using products at home and work get the data needed for informed choice, and researchers can spot potential long-term health effects. Open information builds real safety. Publishing these details, instead of leaving them buried, empowers every hand that touches the product.
Building Trust
Chemical structure and molecular weight do more than fill space on a technical sheet. They open doors for prevention, informed consent, and targeted treatment. In the end, these numbers and diagrams build the bridge from factory or pharmacy all the way to safe use in real lives.
Why New Solvents Matter
The world of chemistry moves fast. Each year, researchers bring new compounds into focus, searching for options that can handle tough jobs. Many of these jobs revolve around replacing traditional solvents, which often create health hazards, flammability issues, or environmental messes. For years, petroleum-based solvents ran the show. Finding safe, efficient, and eco-friendly replacements interests chemical manufacturers, environmental scientists, and even the pharmaceutical folks.
People talk a lot about ionic liquids these days. An ionic liquid doesn’t look like a salt, but under the microscope, its building blocks behave in a similar way. Its components are ions, yet it stays liquid around room temperature. This trait lets researchers handle chemicals more safely, tuning process temperatures and pressures. At the same time, many ionic liquids hardly evaporate, limiting inhalation risks and pollution.
What’s Essential for an Ionic Liquid or Solvent
Any compound pitched for solvent use needs to bring the right features to the table. It can’t explode, corrode the pipes, or mess up the product’s purity. Ionic liquids often score higher in safety and stability. On the downside, some ionic liquids stay pricey, require special handling, or release tough-to-break-down compounds after use. A new candidate gets a lot of attention when it promises to side-step these headaches.
I worked with a start-up aiming to swap out chlorinated solvents for a new ionic compound. Early tests ran great in the lab: high solubility, low odor, zero flammability. But, under industrial conditions, stubborn residues grew. Equipment cleaning costs soon doubled. What looks neat on paper can disappoint during scale-up. This shows that new solvents need full performance testing—not just simple lab beakers, but reactors, vats, and waste streams.
The Search for a Better Way
Newer ionic liquids offer important advantages over their conventional rivals. They dissolve a wide range of molecules, help speed up chemical reactions, and steer reactions in certain directions. For industries making high-value chemicals or greener pharmaceuticals, these new liquids give real-world benefits. They also make recycling simpler, since some can be re-used several times with the right regeneration step.
Regulations, particularly in Europe and North America, keep cracking down on hazardous solvents. Any company looking to introduce a new chemical gets grilled over safety. Does the compound break down in water or soil? Could an accidental spill spread quickly? What is its toxicity to humans and wildlife? Developers now include eco-data alongside melting points and solubility charts. The search for greener solvents won’t end soon. Recent European Union standards require more data disclosure about toxicity, persistence, and bioaccumulation risk. Transparency builds trust, for both investors and the public.
Potential Solutions: From Lab to Factory Floor
Scale-up walks hand in hand with thorough vetting. This isn’t just about chemistry—big-picture thinking involves people, machines, and the environment. Pilot projects bridge the gap between university labs and full-blown chemical production. Early collaboration with regulatory bodies can head off compliance setbacks. Toxicology labs check degradability and potential health impacts. Manufacturing teams stress-test pumps, seals, and pipelines. Waste management experts jump in to confirm the spent compound can be either recycled or safely neutralized.
If a new compound shines as both an ionic liquid and a solvent, researchers should push for open data-sharing. Cross-industry partnerships can shorten the time from discovery to industrial adoption. Chemists will keep looking for new solutions, as real change depends on teamwork from labs, regulators, process engineers, and the public. Every safer, cleaner compound offers a win: fewer hazards, healthier workers, and progress toward a greener chemical industry.