Understanding 1,3-Dibutylimidazolium Hexafluorophosphate: Insights and Future Directions
Historical Development
A little over two decades ago, chemists began digging deep into the field of ionic liquids as they chased new solvents for greener reactions and easier product isolation. Among the compounds that emerged, 1,3-Dibutylimidazolium hexafluorophosphate quickly attracted interest. Synthetic efforts in the late 1990s unlocked the door, with researchers tuning imidazolium rings to explore their stability and dissolving power. Scientists realized that swapping out methyl or ethyl for butyl on the imidazole backbone led to improved chemical resistance and a wider temperature window for use. As more attention landed on sustainable chemistry, this compound shifted from being a specialized laboratory material to a staple in many advanced applications, often referenced as "BBIM PF6" within scientific circles.
Product Overview
Digging into the actual product, 1,3-Dibutylimidazolium hexafluorophosphate stands out with its specific combination of imidazolium cation and hexafluorophosphate anion. Chemists value it for its stability under a surprisingly broad range of laboratory settings. Unlike early ionic liquids that were sluggish or prone to breakdown, this salt holds together, letting researchers use it as a solvent, electrolyte, or catalyst medium. The practicality comes through in viscosity that falls in a sensible range and a melting point that lets it remain liquid during many experimental setups. For chemists and engineers, the combination of thermal stability and low volatility truly matters, allowing safer handling than many traditional solvents.
Physical & Chemical Properties
1,3-Dibutylimidazolium hexafluorophosphate presents as a colorless to pale yellow liquid at room temperature, though colder storage pushes it toward a waxy solid state. A molecular weight near 382.32 greets those doing stoichiometric calculations, while a boiling point that barely climbs above 300°C draws curious eyes. The ionic nature delivers a high polarity; it dissolves a range of organic and inorganic compounds, which finds heavy use in both research and pilot plant work. Water solubility remains quite low, which helps in separating it from aqueous phases but also means care must be taken to prevent unwanted hydrolysis or moisture absorption from the air. Strong conductivity in the liquid state offers real value for battery and electrochemical uses.
Technical Specifications & Labeling
Suppliers distribute the product under a variety of catalog numbers, often highlighting its purity at 98% or greater. Chemical labels should spell out its proper IUPAC name: 1,3-dibutyl-1H-imidazol-3-ium hexafluorophosphate. Labels usually include a batch number, date of manufacture, and storage instructions, with a clear warning about potential moisture sensitivity. Handling data informs users about its density, which hovers around 1.18 g/cm³ at room temperature. The compound’s unique identifiers, such as CAS number 246525-35-9 and relevant UN shipping codes, underpin safety and traceability for compliance with global transport and workplace standards.
Preparation Method
The synthesis route begins with 1-butylimidazole, which reacts with n-butyl bromide under carefully controlled temperatures to create 1,3-dibutylimidazolium bromide. After purification, an ion-exchange reaction follows, with potassium hexafluorophosphate introduced to swap out the bromide ion for PF6-. The product precipitates in an organic solvent like dichloromethane, followed by filtration and vacuum drying. Meticulous control of stoichiometry and temperature helps keep side products low, while repeated washing ensures the removal of inorganic salts. This process, often scaled for academic or industrial needs, highlights the vital balance between yield and purity in specialty chemical manufacturing.
Chemical Reactions & Modifications
This ionic liquid offers an impressive resilience, with the PF6- anion resisting decomposition across many reaction conditions, from acidic to mildly basic. The imidazolium ring resists nucleophilic attack but still participates in hydrogen bonding and π-stacking, enabling applications as a reaction solvent or even as a phase transfer catalyst. Researchers have introduced functional groups onto the butyl side chains, aiming to tweak solubility or tailor its behavior for certain chemical tasks. While the parent form stays unreactive under most conditions, choosing alternative anions opens even more possibilities in customizing reaction environments, which matters in pharmaceutical and materials science explorations.
Synonyms & Product Names
Popular synonyms for this compound include 1,3-Dibutylimidazolium PF6, BBIM PF6, 1,3-Dibutyl-1H-imidazol-3-ium hexafluorophosphate, or even simply as an “imidazolium ionic liquid” in shorthand notes. For regulatory documentation, suppliers stick to standardized nomenclature or provide a list of synonyms on safety data sheets to ensure buyers know exactly what stands in the bottle, crossing linguistic or market-specific naming conventions.
Safety & Operational Standards
Anyone working with 1,3-Dibutylimidazolium hexafluorophosphate learns early that the hexafluorophosphate anion, while chemically stable, can break down in the presence of strong acids or high heat to form toxic PF5 or HF. Proper laboratory practice means running all reactions in well-ventilated hoods and storing the material in tightly closed containers to minimize moisture uptake and decomposition. Direct skin or eye contact should be avoided, with gloves and goggles included as standard gear. Waste disposal must follow hazardous waste protocols due to the PF6- ion’s persistence in the environment. Companies and universities often run routine training sessions on handling ionic liquids, sharing incident reports to foster safer workplaces.
Application Area
1,3-Dibutylimidazolium hexafluorophosphate has carved a spot in several high-tech fields. Electrochemists look to it for electrolytes in batteries and supercapacitors, citing its high ionic mobility and wide voltage window. Chemical engineers use it as a reaction medium for transition metal catalysis, where its low volatility supports cleaner product isolation. Analytical chemists employ it in sample extraction workflows, separating organic pollutants from water or soil with minimal cross-contamination. Recently, interest has surged in using it for transforming lignocellulosic biomass, where it helps modify plant polymers into fuel alternatives under mild conditions. This cross-disciplinary appeal comes from its balance of stability, solubilizing power, and manageable cost.
Research & Development
Innovation rarely slows, and the community around ionic liquids keeps pressing forward. Teams in academia and industry push to expand the compound’s uses, experimenting with composite electrolytes for next-generation batteries, greener solvents for pharmaceutical synthesis, and absorbents for pollutant capture. Research often zooms in on reducing toxicity or improving biodegradability, inspired by regulatory momentum and growing environmental awareness. For instance, new derivatives have been engineered with shorter or more polar alkyl chains, trading off some thermal stability for easier breakdown in wastewater systems. Pilot projects apply BBIM PF6 in process intensification, shrinking plant footprints and cutting hazardous solvent needs. The push for circular chemistry sees it deployed as both solvent and reagent recycler in closed-loop manufacturing.
Toxicity Research
Toxicologists study 1,3-Dibutylimidazolium hexafluorophosphate with a careful eye, since both the cation and the PF6- anion raise questions. Research points to its low acute toxicity for short-term lab exposure, though chronic effects remain less certain. PF6- can slowly hydrolyze under acidic or humid conditions, forming HF, which poses health hazards through inhalation and contact. Some studies have flagged concerns about persistent bioaccumulation of fluorinated species in aquatic systems, prompting further examination of its lifecycle uses and end-of-life disposal practices. Investigators recommend minimizing release into wastewater, exploring alternative anions or biodegradable cation frameworks, and calling for transparent reporting of workplace exposures and environmental monitoring.
Future Prospects
The next decade holds a mix of challenges and possibilities for BBIM PF6. Battery technology continues to need tailored electrolytes to support electric vehicles and renewable grid storage, making this compound and its relatives strong candidates for further R&D investment. Pharmaceutical companies scout for solvents that simplify complicated syntheses without toxic legacy, and BBIM PF6’s stability and solvent power keep it on the shortlist. Environmental pressures are likely to steer development toward more biodegradable or less persistent variants, with green chemistry teams hunting for replacements to the PF6- anion. Large-scale applications, from carbon capture to biopolymer processing, look promising as manufacturing costs fall and regulatory comfort grows. Collaboration between industry, academia, and regulatory bodies will be crucial in turning these prospects into safe, economically viable technologies.
The Rise of Ionic Liquids in Modern Chemistry
Back in the day, finding truly versatile solvents for chemical reactions or materials processing always posed challenges. Toxicity, volatility, even the cleanup process—those issues slowed things down in labs and on factory floors. Over the years, ionic liquids carved out a niche, especially ones like 1,3-dibutylimidazolium hexafluorophosphate. It’s not just another fancy compound; it showed chemists and engineers new options beyond traditional solvents or electrolytes.
Electrochemical Applications that Transform Devices
Plenty of batteries and capacitors still run on tried-and-true electrolytes, but ionic liquids with properties like non-flammability and low vapor pressure became attractive. 1,3-dibutylimidazolium hexafluorophosphate has the kind of thermal and electrochemical stability that opens doors. I worked with physicists on prototype devices who looked for safer, longer-lasting alternatives to conventional lithium salts. This ionic liquid delivered. In supercapacitors, it pushed working voltages higher and kept degradation in check. It also made lithium-ion batteries safer, which matters for anything from cell phones to electric vehicles.
With higher ionic conductivity and the ability to keep moisture away, this material lets engineers design denser, more reliable storage. That translates to real world impact for grid storage or devices that see heavy cycles. Its immiscibility with water also helps extend component lifetimes.
Solvent Power in Chemical Synthesis and Catalysis
Every industrial chemist learns the headaches caused by volatile organic solvents: health worries, flammability, emissions regulations. Shifting to ionic liquids like 1,3-dibutylimidazolium hexafluorophosphate allowed safer lab environments. With few emissions and good stability, reactions can stay cleaner and often produce higher yields. This compound proved handy for transition metal catalysis, coupling reactions, and extracting trace metals. Researchers at several universities reported better selectivity for catalytic processes thanks to its solvation properties.
It doesn’t evaporate, so chemists can recover and reuse it after reactions. Over time, that saves money and cuts down hazardous waste. Refineries and pharmaceutical labs caught onto this trend as green chemistry goals tightened.
Precision Cleaning and Metal Processing
In microelectronics manufacturing, removing residues and cleaning tiny features relies on precision. Conventional solvents can leave films or damage materials. 1,3-dibutylimidazolium hexafluorophosphate’s unique ionic nature solves those issues. It dissolves residues where regular options fall short but doesn’t attack sensitive surfaces. Semiconductor plants see fewer defects thanks to its gentle but effective cleaning. That upside drives higher yields and faster production turnaround.
Metal-processing industries also put it to work. They use ionic liquids in electrodeposition or refining schemes for rare metals and alloys. This ionic liquid’s thermal stability stands up to harsh environments, making it a go-to for extracting and purifying metals like gold and palladium. These processes rely on specialized chemistry, and ionic liquids often outperform water-based alternatives.
Environmental Considerations and Safer Handling
The push for safer, cleaner industrial chemistry pushed people to rethink everything from solvents to waste streams. 1,3-dibutylimidazolium hexafluorophosphate offers lower hazards than a lot of volatile organics, but not all ionic liquids are perfect. Researchers continue looking into its persistence and toxicity. Companies using it invest in closed systems and robust recycling methods to minimize emissions or accidental loss.
Ongoing research explores biodegradable or even less toxic alternatives, but the role of this ionic liquid is tough to beat for now. As labs and industries look to cut waste and streamline processes, experience keeps showing that 1,3-dibutylimidazolium hexafluorophosphate meets a real need—balancing performance, safety, and adaptability across fields that rely on chemistry to keep moving forward.
Looking Closely at the Chemistry
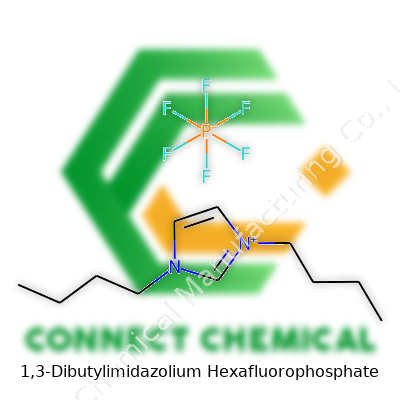
Let’s talk about 1,3-Dibutylimidazolium Hexafluorophosphate, a salt that keeps popping up in research circles. You see it abbreviated as [C4C4Im][PF6]. From the outside, the name looks like a mouthful, but digging into the structure helps things click. The heart of this compound lies in its imidazolium ring, a five-membered ring with two nitrogen atoms sitting across from each other. It’s a backbone chemists lean on for building ionic liquids.
This molecule swaps typical hydrogen atoms at the ring's first and third positions for straight-chain butyl groups (C4H9–). Adding these long alkyl branches cranks up the compound’s size and cuts down on its water solubility. The imidazolium part forms the cation, while hexafluorophosphate (PF6−), with its phosphorus center tightly wrapped by six fluorine atoms, supplies a stable and bulky anion.
Tallying Up Molecular Weight
Numbers matter in the lab. The chemical formula for the cation stands as C11H22N2+. The anion sits as PF6−. Grabbing a calculator, you total up the molar mass like this:
- Cation: Carbon (C) × 11 = 132.11 g/mol; Hydrogen (H) × 22 = 22.18 g/mol; Nitrogen (N) × 2 = 28.02 g/mol
- Anion: Phosphorus (P) = 30.97 g/mol; Fluorine (F) × 6 = 113.99 g/mol
Add these together, and the molecular weight lands around 327.5 g/mol (cation) plus 144.96 g/mol (anion), giving a total near 472.46 g/mol. Precision counts in experiments, so checking each element’s actual atomic weight can nudge this a hair up or down, but most sourcing data echo this figure.
Why Are People Interested?
I spent a chunk of time in a graduate lab flooded with talk of ionic liquids. This compound, with its specific mix of structure and charge, shows up on the shortlist for green chemistry solutions. It’s all about solvents that don’t evaporate like traditional organics. Labs reach for 1,3-dibutylimidazolium hexafluorophosphate during extractions or electrochemical work. Its low vapor pressure means no stinging fumes, which makes a huge difference on a busy bench.
Being both stable and able to dissolve a variety of things draws industry and research teams alike. They use these ionic liquids in areas stretching from batteries to organic reactions. I’ve seen how a well-designed ionic liquid can sidestep old problems connected to volatile solvents — less risk, cleaner air, less flammability.
Tackling Issues and Looking Ahead
No chemical is perfect. Concerns trail along with hexafluorophosphate-based liquids. These anions can drop toxic by-products if mishandled or heated under harsh conditions, especially HF (hydrogen fluoride). Exposure protection and proper waste strategies play a big role here. In my experience, nobody wants a cap of HF acid opening up near their work area. Solid safety training and tightly closed waste containers keep problems small.
Sustainability teams look for ways to improve further. Research groups have started exploring replacement anions and biodegradable imidazolium salts. Finding alternatives means balancing performance with safety and scalability, but the effort is well worth it. Seeing peers tinker with less hazardous options feels hopeful, signalling the industry won’t stand still. Ionic liquids, once a curiosity, shape up to be tools for the next wave of cleaner processes, as long as the health and environmental questions stay front and center.
Direct Exposure Risks
Few people outside specialized labs ever cross paths with 1,3-Dibutylimidazolium Hexafluorophosphate, a salt often used in research for batteries and green chemistry. This isn’t table salt or everyday household material; it’s an ionic liquid where a simple mistake could spell trouble. My first encounter came in a university lab. Gloves, goggles, and lab coats weren’t options but essentials. The compound may not hit with the fury of some acids, but it carries risks. Direct skin contact can cause irritation. Breathing in the dust or vapor risks lung trouble. I’ve heard stories: a friend once knocked over a bottle, and a whiff sent him into a coughing fit for hours. You don’t forget lessons taught by discomfort.
Smart Storage
Storing this chemical asks for a little respect and a lot of planning. Moisture ruins it, so it sits in sealed glass bottles away from water sources. The hexafluorophosphate part doesn’t get along with water and can form hydrofluoric acid, nasty stuff that eats through skin and bone. I routinely check containers for cracks or leaks. A dry, well-ventilated space avoids the risk of unwanted reactions if a spill happens. A locked chemical cabinet, far from food or drink, makes a lot of sense if anyone else works nearby. I always keep a spill kit handy. Not using one yet? Now’s the time to change that.
Personal Protective Equipment (PPE)
Long sleeves, thick gloves, chemical-splash goggles. Every layer helps. No one feels comfort in latex or nitrile on a summer day, but burns and rashes feel worse. Impermeable gloves last longer and keep out liquid seepage. I double-check goggles for cracks, and if my lab coat has holes, out it goes. Risks don’t announce themselves; protection has to be proactive. After each experiment, I wash exposed skin—the lather stays on for the full twenty seconds, every time.
Ventilation and Fume Hoods
Fume hoods keep any accidental dust or vapor in line. Labs with poor airflow let small leaks linger and settle in lungs, not just noses. An exhaust vent over the workspace pulls air away and traps it in filters. Not once have I worked with this chemical outside a working hood. If the fan dies, so does any work with hazardous powders.
Emergency Readiness
Some chemicals test your reflexes. Safety showers and eyewash stations stay within arm’s reach. Every spill gets reported and cleaned immediately. Neutralizing agents for hydrofluoric acid, like calcium gluconate, should live nearby. Accidents happen, but quick action limits the damage. A first aid kit close to hand never seems dramatic until you need it.
Waste Disposal
Lab waste isn’t just garbage. Waste from compounds like this one goes in clearly labeled, sealed containers. Disposal follows rules set by local regulators, because pouring it down the drain threatens people and environment. Every month, our team audits old containers and logs every chemical, so nothing nasty gets forgotten at the back of a shelf.
Common Sense, Reinforced by Respect
Sharing a workspace calls for stronger habits. Label every sample, train new colleagues with patience, never eat or drink where chemicals live. Good practice builds a safeguard that no rulebook alone can match. Over the years, I’ve seen colleagues avoid close calls not through luck, but mindful habits. Scientific know-how means little without care for yourself and others. That’s what real safety means.
Understanding the Real Risks
Most lab chemicals bring their own set of risks. I learned pretty early in my lab days that not paying respect to storage rules often results in awkward clean-ups or worse, health scares. Take 1,3-Dibutylimidazolium Hexafluorophosphate as a clear example. This ionic liquid isn’t a simple bottle of vinegar tucked on a high shelf. Folks don’t realize how moisture and temperature shifts can mess with stability and safety.
Practical Storage Tips Drawn from the Bench
Start with location. A solid chemical storage room beats a regular supply closet every time for this. Humidity plays tricks, so a room with controlled temperature and dryness helps keep this chemical from degrading or reacting with water in the air. You definitely wouldn’t keep it near a chemical sink or windows, where sunlight and temperature changes are regular guests.
Standard practice in my labs calls for a tightly sealed, clearly labeled container. I've seen students recap bottles lazily and come back next week to a sticky mess, especially with ionic liquids. Glass containers with teflon-lined lids solve most problems here, stopping leaks and blocking any water vapor. Skip plastics that might react or let things through. Never take for granted that one bottle fits all—compatibility charts exist for a reason, and checking the manufacturer’s recommendations can save a ton of trouble.
Fire Hazards and Chemical Friends & Foes
This ionic liquid does not set itself ablaze, but combining it with strong acids or bases without proper containment invites risk. Storing it away from oxidizing agents keeps things safe. I don’t keep it near nitrate or permanganate supplies for exactly this reason.
Temperature matters—a lot. I keep it below 30°C, based on both the safety data and my own experience with chemical stability. Refrigeration isn’t necessary, but don’t let jars sweat on a hot summer shelf. Wrapping containers in aluminum foil blocks the light, keeping the compound from breaking down over time.
Using Labels and Inventory as Safeguards
A small investment of time in labeling saves hours later searching or—worse—accidental mix-ups. At my last job, I watched a colleague mix two clear liquids, convinced each was just water. They weren’t. Bold, smudge-proof labels covering contents, concentration, date received, and hazard symbols keep everyone on the same page, even when team members change.
Inventory logs aren’t just bureaucracy. Tracking who took out the last bottle and when can prevent someone from using an out-of-date or decomposed sample that’s spent months on the wrong shelf. This also helps spot leaks in storage systems—if it’s vanishing fast, there’s probably a spill.
Handling Spills and Training Staff
Accidents test a system’s strength. Absorbent pads and nitrile gloves need to stay close by. I encourage routine spill drills, as even experienced team members freeze up faced with an uncommon chemical. Posting up-to-date safety sheets and emergency numbers at eye level lets anyone access vital info in a pinch. In my experience, periodic training sessions keep processes fresh in everyone’s mind and prevent complacency.
What Matters Most
Respect for chemicals grows with each mishap avoided. Storage isn’t just about following textbook rules. The right approach protects people, keeps materials pure, and saves research from setbacks. With 1,3-Dibutylimidazolium Hexafluorophosphate, staying vigilant with storage practices pays off in reliability and safety long-term.
What Makes Compatibility a Real Concern?
Chemists look for chemicals that play well with others, especially in processes where new materials or solvents offer better performance. 1,3-Dibutylimidazolium hexafluorophosphate, a mouthful for sure, shows up in conversations about ionic liquids used in batteries, catalysis, and extraction. Folks want to know if it mixes safely and effectively with different compounds, or if it throws up roadblocks in the lab.
Digging Into the Science
I have seen colleagues torn between traditional solvents and compounds like this one. The draw comes from its stability, low melting point, and ability to dissolve a wide range of materials. But stories of unexpected reactions, poor solubility, or degradation pop up enough to keep everyone on their toes. It’s not just about mixing; it’s about how the chemical holds up under heat, air, water, and aggressive reagents.
Take water, for example. Many ionic liquids lose their cool with trace moisture. In this case, hexafluorophosphate anions can break down, releasing acidic products. That spells trouble if someone needs precise control over acidity or long lifespans in batteries. It’s a real limitation, not an abstract one—degradation means money lost and experiments derailed.
Organic solvents can be another sticking point. Compatibility often hinges on polarity and whether the other compound pulls double duty as a reactant or just a bystander. Nitriles, alcohols, and halogenated solvents all bring different baggage. In the wrong mix, you might run into sluggish reaction rates or full-on precipitation. Colleagues in green chemistry often remind me that chasing greener methods isn’t as simple as swapping out one solvent for another. The details matter if you want safe, scalable processes.
Too Many Promises, Not Enough Follow-through
Ionic liquids often get hyped as miracle ingredients. Real-world experience pushes back. Take electrochemistry. Researchers have found that 1,3-dibutylimidazolium hexafluorophosphate can support stable, wide voltage windows for some electrodes but isn’t immune to degradation or product crossover from other salts. All it takes is the wrong counterion or trace impurities to see erratic current readings or fouled electrodes. This isn’t some rare lab trick—it happens often enough that engineers take pains to purify reagents and monitor conditions constantly.
Industry users want clear data on reactivity. Given the cost and complexity, it’s frustrating to hit stumbling blocks after the fact. I’ve seen teams run into persistent foaming, unexpected byproducts, and corrosion issues on plant equipment. A quick scan of the literature can offer some comfort, but there’s always uncertainty unless teams run their own compatibility trials.
How To Move Forward Without Rose-Colored Glasses
People working with ionic liquids need solid guidelines, not just glowing testimonials. I tell colleagues to scrub every material they plan to add to a system, looking for surprises in stability and toxicity. Beyond checking datasheets, it pays to talk to others who have run similar processes. Share notes, ask hard questions, and run those ugly control experiments before scaling up.
Bumping up peer-reviewed studies helps. More real-world reporting—failures and all—can give engineers and chemists a toolkit for smarter choices. Suppliers can chip in by offering not only pure product but also technical support grounded in truth, not just slick marketing. Cautious testing, solid collaboration, and honest communication keep progress steady, not just flashy.