1,3-Diethylimidazolium Chloride: A Practical Perspective on Its Journey, Properties, and Role in Science
Historical Development
Chemists spent most of the 1900s using organic solvents that created headaches for both people and the planet. Around the late 20th century, the spotlight started shifting to ionic liquids. These salts remain liquid near room temperature, opening doors that once seemed tightly locked. 1,3-Diethylimidazolium chloride emerged from this rethinking of the “rules” for solvents. Researchers wanted a liquid that could handle tough jobs without evaporating into thin air or catching fire easily. The imidazolium story began with the basics: look at the imidazole ring, swap out those hydrogen atoms at the nitrogen positions for simple alkyl groups like ethyl, and suddenly a new liquid was on the shelf. Over decades, these tweaks created a family of ionic liquids, with 1,3-diethylimidazolium chloride gaining its own reputation for unique properties and reliability across various laboratory and industrial tasks. Scientists documented its stability and growing use in well-respected publications, pushing this compound onto a path toward mainstream use.
Product Overview
1,3-Diethylimidazolium chloride presents itself as a nearly colorless or pale white crystalline solid, sometimes melting into a clear, low-viscosity liquid at room temperature. Unlike typical table salt or volatile solvents, this material doesn’t easily evaporate or catch fire, making it easy to store and ship. Its main appeal lies in being an efficient, nonvolatile solvent and a practical starting material for further synthesis. Chemists usually buy it in tightly sealed, moisture-proof containers since it tends to want water from the air, and the pure product often defines the fate of an experiment or application. The chemical industry, research labs, and startups in green technology all understand that starting with a well-prepared batch sets the stage for successful chemistry down the line.
Physical & Chemical Properties
Looking at its molecular structure, 1,3-diethylimidazolium chloride piles on the benefits of ionic liquids. This compound has a melting point around 74–77°C, keeping it stable at common working conditions. It dissolves readily in polar solvents like water, methanol, and acetonitrile, but resists mixing with non-polar materials such as hexane or heptane. The salt attracts and holds onto water molecules from the air if left exposed, something anyone storing a bottle has learned rapidly. A moderate electrical conductivity paired with low vapor pressure creates a useful niche: it works neatly in electrochemical setups, and evaporative loss never raises eyebrows. While some organic solvents set off alarms with fumes and flammability, 1,3-diethylimidazolium chloride’s stability and safety grant it a credible spot for green chemistry approaches.
Technical Specifications & Labeling
Suppliers label this compound by its systematic name, 1,3-diethyl-1H-imidazol-3-ium chloride, often referencing the CAS registry number 64899-02-3. Bottle labels flag its purity, usually above 98%. Some bottles carry warnings about hygroscopicity and safe handling. The material should travel in airtight, registered containers, especially when ordered for laboratory or pharmaceutical use. Batch numbers, lot numbers, and certificates of analysis trail each shipment, rooting out uncertainty for those running sensitive reactions. Reliable supply chains hinge on customers knowing what sits inside their bottle, and regular audits from procurement teams dissect these labels before a substance ever reaches the bench.
Preparation Method
Most chemists prepare 1,3-diethylimidazolium chloride with a straightforward alkylation strategy. Mixing imidazole with ethyl chloride and a mild base in a dry, inert atmosphere launches the desired reaction, forming the quaternized imidazolium salt as a precipitate or oil. The process happens over several hours, usually at slightly elevated temperatures. Technicians wash the crude product repeatedly with solvents such as ethyl acetate or acetonitrile, removing any unreacted starting materials. Some adaptations tweak the reaction, switching out starting compounds or purification protocols depending on the equipment or downstream needs. Moisture control from start to finish plays a big role, as traces of water can throw off yields and transform an otherwise simple reaction into a headache.
Chemical Reactions & Modifications
1,3-Diethylimidazolium chloride serves as a launch pad for fresh research. Chemists swap out the chloride for other anions in metathesis reactions, creating custom ionic liquids for specialized needs—sometimes trading the chloride for tetrafluoroborate, hexafluorophosphate, or bis(trifluoromethanesulfonyl)imide. Nucleophiles target specific carbons or hydrogens on the imidazolium ring, leading to modification of the molecule for desired solubility or reactivity. Electrochemistry labs harness its inherent conductivity in battery or sensor projects. Researchers studying catalysis or phase transfer reactions rely on the salt’s exceptional stability in both acidic and basic conditions, pushing forward a variety of new methodologies in organic synthesis or separation science.
Synonyms & Product Names
Chemists and suppliers may refer to this compound as 1,3-diethylimidazolium chloride, 1,3-diethyl-1H-imidazol-3-ium chloride, or DEImCl. Some catalogs prefer abbreviations, such as [Et2Im]Cl. Names change across regions and industries, yet a thorough look at the CAS number or structural diagram usually clears up any confusion during procurement or literature review. Clear labeling saves time, especially in multinational labs juggling different suppliers or translation challenges.
Safety & Operational Standards
No chemical is truly “safe” in careless hands. 1,3-Diethylimidazolium chloride usually avoids the drama found with classic solvents or volatile materials, but basic chemical hygiene matters tremendously. Users should wear gloves, goggles, and laboratory coats to prevent skin or eye contact. Because the powder absorbs water from the air, careful storage in sealed bottles away from humidity or incompatible chemicals protects the quality and integrity of the batch. Washing hands after use and working in a well-ventilated area make sense for nearly any chemical, this one included. Standard operating procedures underscore the importance of labeling every aliquot and tracking stock levels, since mislabeling or mix-ups can hurt both research and personnel health. The chemical industry and regulatory agencies keep labs on track with regular reminders—not just to follow these protocols, but to continually check that best practices evolve alongside new findings.
Application Area
Academic and industrial settings turn to 1,3-diethylimidazolium chloride for tasks that demand more than old-fashioned solvents or catalysts. Electrochemists find this compound reliable in supercapacitors and battery systems, tapping its conductivity and stability for cutting-edge devices. Green chemists take advantage of its ability to dissolve cellulose and other tough polymers, skipping the need for harsh acids or toxic reagents. This ionic liquid has reshaped extraction and separation workflows, replacing volatile organics with safer options. Some pharmaceutical and agricultural labs experiment with the salt as a reaction medium, hoping to boost yields or control side-products. Innovations in biofuel processing, waste recycling, paper and pulp modification, and organic synthesis all tip their hats to the unique role of ionic liquids like 1,3-diethylimidazolium chloride.
Research & Development
Scientific progress depends on curiosity paired with methodical trial and error. Over the past decade, research teams have tweaked the fundamental structure of 1,3-diethylimidazolium chloride to optimize solubility, thermal stability, or interaction with target molecules. Studies on solvent extraction, stabilization of metal complexes, and enhancement of electrochemical properties keep the compound in frequent rotation among journals and patent filings. As new materials demand more sustainable solvents and processes, R&D projects at academic institutions and in industry push the envelope of what this ionic liquid can deliver. Each advance builds on earlier discoveries: from studying basic physical properties to using high-throughput screening with combinatorial chemistry techniques, the incremental innovation around 1,3-diethylimidazolium chloride has produced fresh insight and often challenges long-held assumptions about chemical separations, catalysis, and green technology.
Toxicity Research
For all the upside, every new material demands careful toxicity review. Researchers tested 1,3-diethylimidazolium chloride on bacteria, plant cells, and animal models to check for cell membrane disruption, acute toxicity, and chronic exposure risks. Some studies show modest toxicity to aquatic life at higher concentrations, which means careful disposal and wastewater treatment are part of responsible lab management. Researchers reported lower volatility and environmental persistence compared to common solvents, yet new findings sometimes lead to updated handling guidelines. Health and safety teams review the literature to protect both individual users and ecosystems. Cross-institutional projects with toxicologists, environmental engineers, and regulatory agencies ensure that rising demand for the compound doesn’t outpace the best understanding of its long-term effects. Workers and communities benefit when research stays one step ahead of industrial practice—a lesson older generations learned all too late from toxic compounds like benzene or chloroform.
Future Prospects
Looking ahead, 1,3-diethylimidazolium chloride sits near the center of discussions about green chemistry and next-generation materials science. Engineers search for ways to recover and recycle this compound to reduce process costs and lighten the environmental load. Synthetic chemists keep tinkering, searching for new derivatives that fine-tune solubility, melting points, or chemical compatibility. Regulators and manufacturers collaborate to build best practices for handling, waste management, and exposure monitoring. Scale-up from the lab bench to factory floor requires constant troubleshooting: every setback becomes a data point for future improvement. Academic partnerships help translate fundamental findings into commercial solutions, guiding students and postdocs into careers at the edge of chemical technology. Instead of a brief moment in the spotlight, 1,3-diethylimidazolium chloride continues to build its relevance, responding to each challenge thrown its way with a mix of reliability, adaptability, and scientific curiosity.
Why Chemists Reach for This Compound
1,3-Diethylimidazolium chloride gets plenty of attention behind lab doors, mostly because of its role as an ionic liquid. In my work with chemical engineering teams, this compound gathered fans for its unique mix of low melting point and stability. Researchers use it as a solvent where water or traditional organic solvents won’t do. It stands up to the test in processes where regular compounds break down or interfere with the chemical reactions.
The pharmaceutical field often turns to 1,3-diethylimidazolium chloride during drug synthesis and purification techniques that require more than just heat and time. Its solubilizing power opens up routes for making molecules without resorting to toxic or explosive materials. That matters in a world still haunted by chemical spills and residue. You see, in labs where green chemistry gets more than lip service, this type of ionic liquid keeps energy costs low—no need for cranking up the heat—because it stays liquid at lower temperatures.
Beyond the Test Tube: Industrial Impact
The story doesn’t end at the bench. Factories that need efficient ways to separate chemicals lean into processes that use these imidazolium-based solutions. During biomass processing, for instance, 1,3-diethylimidazolium chloride helps break down plant material to create biofuels, specialty polymers, and even textiles from cellulose. My own experience analyzing pilot projects for sustainable materials showed how this compound can replace harsher acids or bases.
In electrochemistry, technicians use it for electroplating and battery technology. Good ionic conductivity and solid thermal stability put it in the running for new generations of batteries and capacitors. Researchers test this stuff in supercapacitor and lithium-ion prototypes, aiming for batteries that last longer and charge faster. Its low volatility also means less fire risk, which keeps both labs and large-scale factories safer.
Safety and Sustainability Concerns
People sometimes worry about what happens after these chemicals leave the lab. Ionic liquids don’t always break down easily, and their effects on ecosystems remain under study. Reports from environmental chemists in Europe and Asia highlight cases where improper disposal led to issues for aquatic life. Responsible teams make sure they’re recovered and reused. The chemical industry pushes for processes that recycle these liquids or swap them for even greener options when real-world data shows long-term harm.
Another challenge revolves around cost. Large-scale projects sometimes struggle with the price tag on ionic liquids like 1,3-diethylimidazolium chloride. Research into simpler syntheses offers hope. As scientists get better at making these compounds in bulk, prices could drop, making greener chemical processes more accessible to small and medium businesses.
Looking Ahead
Colleagues in academia and industry see 1,3-diethylimidazolium chloride as part of a wider move toward sustainable chemistry. Its ability to dissolve stubborn materials, support novel batteries, and cut back on dangerous solvents gives it an edge. With careful research into long-term health and environmental impacts, along with smarter reuse practices, this ionic liquid could help shape a safer and cleaner chemical future.
Understanding Its Shape and Function
The first time I came across 1,3-Diethylimidazolium chloride, I was sitting in a sunlit lab surrounded by the faint scent of solvents and coffee. The imidazolium family often pops up in conversations about ionic liquids. These compounds turn out to be far more than textbook curiosities. Their roles reach into everything from advanced batteries to green chemistry. Getting to know their structures helps us see how science moves from textbooks to things that matter in day-to-day life.
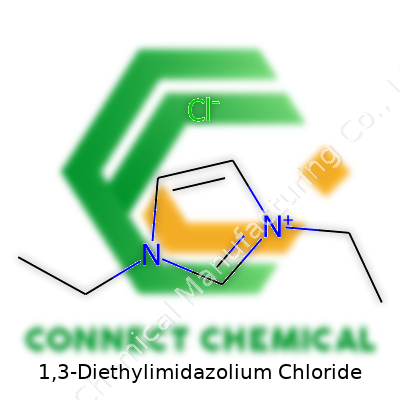
Picture a five-membered imidazole ring—a playground for electrons, shaped something like a pentagon. Two nitrogen atoms break up the carbon chain, giving this ring a unique chemistry. For 1,3-diethylimidazolium, each nitrogen picks up an ethyl group. So, instead of simple hydrogens, those spots are now capped off with two-carbon chains. That small swap has big consequences for how the molecule behaves. The overall charge shifts. Suddenly, this compound wears the badge of a cation (positively charged), hungry for a counter partner. Chloride steps in, bringing negative charge and evening things out. So you get 1,3-diethylimidazolium chloride—a two-piece puzzle of a molecule.
Why This Structure Matters
In chemistry, small changes light up new possibilities. The ethyl groups on the nitrogens nudge the molecule toward interesting behaviors. Unlike standard salts that stack up tightly, these bulkier organic ions refuse to pack so closely. That tweak drops the melting point. The result is a room-temperature ionic liquid—a material that stays fluid even on a cool day and resists evaporation. For chemists pushing for greener solvents or smooth-running electrochemical reactions, this brings fresh hope.
Researchers climbed into the details with X-ray crystallography, mapping the imidazolium cation. Two ethyl groups stretch out from the planar core, giving the structure an organic flair that opens doors in solution chemistry. The chloride ion orbits nearby, carrying its own set of interactions. These “ionic liquids” do away with the flammable fumes tied to volatile organics. They often find their way into batteries, hydrogen fuel cells, and some niche drug delivery systems. Not every story is perfect, though. Their persistence in nature poses challenges—once made, these molecules don’t break down easily. Some can even be toxic if released carelessly. The lesson? Innovation walks hand-in-hand with stewardship.
Responsible Steps Forward
Each time someone picks up a beaker of 1,3-diethylimidazolium chloride, it pays to think about safer handling. Gloves, goggles, and good ventilation remain non-negotiable. Laboratory safety sheets flag potential health signals, catching the attention of everyone nearby. Disposal protocols stay strict to keep local waterways safe. For future progress, material scientists dig into greener synthesis methods and ways to build in easier natural breakdown. That way, benefits in battery life and sustainable chemistry don’t end up costing future generations their clear water and clean air.
1,3-Diethylimidazolium chloride proves that molecular structure echoes much further than a chemistry classroom. It wraps practical science, environmental caution, and new possibilities into a single, strangely elegant five-membered ring topped with ethyl tails and balanced by chloride. There’s always more to learn from the shapes that make up our world.
Looking at the Safety of 1,3-Diethylimidazolium Chloride
People working around chemicals often ask about the real risks behind the long scientific names on the bottle. 1,3-Diethylimidazolium chloride, a type of ionic liquid, tends to show up in research, industry, and some advanced manufacturing because of its unique properties. Yet, the story does not end with its usefulness. The risk side needs some daylight too.
Understanding Its Hazards
Most of us do not have this compound under the kitchen sink, but labs and certain factories handle it regularly. When you look at its toxicity, scientists point out that many ionic liquids—including this one—can hit living cells hard if they spill out of managed spaces. Studies have found 1,3-diethylimidazolium chloride can disrupt cell membranes, damage aquatic organisms, and could harm the people exposed to enough of it. Not everything about these risks is clear because regulators have not thoroughly tested many of these chemicals like they have with more common substances.
Researchers at universities noticed fish and water fleas struggle in waters tainted with low amounts of this compound. A German research team published results showing clear harm to sensitive aquatic species at concentrations that might build up in the environment. It raises the question: Do we know enough before pouring more of these substances into everyday processes?
A Look at Worker Safety
Chemists measure toxicity with numbers like LC50 or EC50, telling you what dose causes half of a test group to suffer or die. For 1,3-diethylimidazolium chloride, these numbers sit in a range that makes safety goggles and gloves a solid requirement in the lab. The risk comes not just from swallowing or breathing it in but through contact—skin absorbs small molecules, and accidents happen even under good supervision. Over time, tiny leaks do add up, especially in places lacking proper fume hoods or training. Even compounds without a sharp odor or strong fumes sometimes pack a punch at surprisingly low doses.
No Silver Bullet Solutions—But There Are Steps Forward
The world keeps looking for newer, greener ways to produce materials, run batteries, and handle chemical reactions. Some say ionic liquids can help, but only if we treat them with real respect and honesty about what they can do to people and nature. Substituting 1,3-diethylimidazolium chloride for less toxic chemicals makes sense where the application allows. Good engineering controls, proper spills training, and up-to-date safety data sheets go a long way too. Companies pressing forward with these tools need transparency, letting the public and regulators see research into effects well before full-scale production takes off.
More funding for toxicology studies and faster sharing of real accident data helps everyone. Years spent cleaning up old chemical mistakes show the hidden cost behind working blind. People benefit when the best minds in chemistry, public health, and industry join forces and keep the full picture in view—efficiency, innovation, and safety too. Reducing environmental risks and workplace accidents never comes from paperwork alone, but from a steady habit of asking hard questions—starting with whether the benefits of chemicals like 1,3-diethylimidazolium chloride really outweigh the hazards.
Understanding the Compound
1,3-Diethylimidazolium chloride shows up in various labs these days, especially in places where research on ionic liquids and greener chemistry solutions takes the spotlight. I’ve worked with similar chemicals and know firsthand how overlooked details in storage can turn into big headaches later. The right approach doesn’t start and stop with just throwing the bottle on a shelf—it requires setting up your space and your routine to respect both the risks and the long-term condition of your supplies.
Keep Stability Top of Mind
Moisture creeps into everything. 1,3-Diethylimidazolium chloride, like other salts, tends to suck water right out of the air. Any batch exposed to humid conditions ends up clumping or forms strange films. A tightly-sealed container makes a world of difference. Glass bottles with proper threaded lids or high-density polyethylene containers both do the trick. For added protection, silica gel packets tossed into storage bins help absorb stray moisture.
Warm rooms cut shelf life short. I’ve seen labs leave sensitive compounds next to heat-producing machines, which speeds up any breakdown or caking. For stable storage, cooler spots away from direct sunlight and heating ducts suit this compound best. Don’t crowd the shelf. Crowding creates pockets where warm air builds up and sticks around.
Label Everything Clearly
More than once in my early days, faded markers and sloppy labels led to confusion between samples. Strong, printed labels bearing the full chemical name and date prevent waste and accidents. If multiple people share the lab, tracking who last handled the jar helps spot issues fast—like a broken seal or an unexpected change in appearance.
Record-keeping matters here, especially because a misplaced or degraded compound disrupts ongoing projects. Even a single contaminated jar can quietly derail months of steady progress or skew experiment repeatability.
Safe Handling Should Be Routine
Chemical safety rules often look tedious, but following them avoids small-scale disasters. Nitrile gloves and lab coats protect skin from direct exposure. Safety goggles keep accidental splashes from turning into eye injuries. I always keep a spill kit stocked—sorbent pads and neutralizing agents prevent tiny mishaps from becoming chemical stains or inhalation risks.
Disposal practices require equal care. Any unused or expired sample goes into designated hazardous waste bins. Sink disposal turns a lab hazard into a plumbing and environmental problem, which responsible professionals want to avoid at all costs.
Training Young Scientists
Fresh faces in the lab may never have worked with a chloride salt like this one. Short training sessions help them recognize warning signs like weird smells or discolored material. Peer mentorship reinforces habits that prevent routine slip-ups—like always resealing bottles and logging personal use.
Community-Driven Solutions
Labs thrive when every member shares responsibility. I’ve seen teams rotate weekly checks of chemical storage, encourage anonymous tips on safety, and invest in digital temperature and humidity monitors. These small changes come from a place of respect for everyone’s health and the quality of the work. Addressing gaps together helps spot risks before they turn into emergencies, and cultivates an environment focused on improvement, not blame.
When chemists bring attention to details—like storage temperature, accurate labeling, and proactive training—accidents drop off and research integrity improves. It isn’t glamorous, but careful, consistent routines safeguard the lab against setbacks and waste, no matter what project lands on the bench next.
Making Chemistry Smoother in the Lab
In the world of chemistry, laboratories want chemicals to pull their weight. 1,3-Diethylimidazolium chloride lands on research benches because it brings a unique twist. As an ionic liquid, it stays liquid at room temperature, skipping the volatility and stink that come with organic solvents. Chemists reach for it during organic synthesis since it often dissolves both salts and organics in a single pot. Years working alongside researchers taught me there’s less glassware cleanup, fumes feel less oppressive, and waste sits friendlier with disposal rules. These practical perks shape real decisions every week across university labs.
Tough Stuff: Biomass Processing
My old classmate got into biofuel research. Her team faced mountains of stubborn plant matter—old wood shavings, corn stalks, anything with lignin. Some ionic liquids, including 1,3-diethylimidazolium chloride, coax lignin and cellulose apart so enzymes can munch the leftovers. This technology may not headline newspapers, but it gets attention for good reason. The chemical keeps the plant’s tough skeleton from blocking enzymes that make sugar and biofuel. A few big papers show that switching over from industrial solvents to these ionic liquids brings less mess, fewer hazards, and more sugar output. These are small moves that echo across the manufacturing world.
Energy Storage Gets a Boost
Batteries don’t get better without new materials. Researchers turn to ionic liquids like this one because it brings stability and low flammability to electrolytes. Once, I met an engineer who tried to handle a lithium battery fire—an experience that stuck with me. Using ionic liquids can avoid that drama; they stubbornly refuse to burn like classic solvents do. Their stability opens doors for next-gen lithium batteries and supercapacitors, especially in tough environments. On top of that, batteries take a beating over time. Some labs saw longer cycle life and less gunk inside cells when they slipped in imidazolium salts.
Supporting Greener Catalysis
Chemical plants used to rely on toxic metals and older solvents for catalysis. These days, folks obsess over greener approaches. 1,3-diethylimidazolium chloride gives metals a chance to mingle with reactants in ways that water or regular solvents just can’t manage. Catalysts work harder and last longer, nudged along by the unique properties of ionic liquids. There’s a steady push to avoid toxic spills, unbreathable fumes, and harsh chemicals. In conversations with folks working at chemical startups, I noticed how small changes in solvent often mean safer work, quick cleanup, and less public pushback if something leaks.
Looking Forward: Cleaner Industry?
Every advance runs into large-scale roadblocks. Many ionic liquids bring high costs and tricky purification steps. Smart scientists and engineers began recycling these liquids, squeezing value from every drop instead of tossing it out. Industry and academia keep testing for which processes truly win out in terms of safety, cost, and environmental health. Countries with stricter controls on pollution seem more eager to adopt such materials, pushing suppliers to lower prices. The quiet influence of 1,3-diethylimidazolium chloride may not spark headlines, but it shows up time and again where chemists, process engineers, and environmental advocates overlap in their dedication to safer and smarter solutions.