1,3-Dimethylimidazolium Hexafluorophosphate: A Practical Perspective
Historical Development
The story of 1,3-Dimethylimidazolium hexafluorophosphate reaches back a few decades, aligning with the broader search for stable, versatile ionic liquids. Back in the late 20th century, a push to find better solvents for electrochemical applications nudged chemists toward compounds like this. Interest really grew when industries realized the drawbacks of volatile organic solvents—explosion risks, toxic vapors, environmental headaches—and started seeking greener and safer options. Research groups operating in Europe and North America gave 1,3-Dimethylimidazolium salts serious attention in the 1990s. Laboratories noticed their non-flammable properties and ability to dissolve a bewildering array of compounds, which meant a chance for cleaner, safer chemistry, especially in applications like metal plating, batteries, and organic synthesis.
Product Overview
1,3-Dimethylimidazolium hexafluorophosphate falls under the category of ionic liquids, unique salts that stay liquid at comparatively low temperatures. This compound has drawn support from both academic and industrial chemists. The imidazolium ring, tagged with methyl groups, partners with the hexafluorophosphate anion to form a salt with useful properties. You’ll find it prepared as a near-colorless liquid or a white crystalline solid, depending on storage and purity. Its role in the chemical industry mostly pivots around effective ion conduction and solvent abilities, especially where water, traditional acids, or organic solvents come up short.
Physical & Chemical Properties
1,3-Dimethylimidazolium hexafluorophosphate draws attention mostly for its low vapor pressure, thermal stability, and relatively wide liquid range. The molecular formula is C5H10F6N2P, and the salt weighs in at about 256 grams per mole. Many appreciate its melting point, usually between 50–65°C depending on purity, and its ability to handle temperatures up to 200°C before breaking down. You notice its density—usually hovering just above water—and its ability to resist ignition, making fire risks less of a worry. Dissolving it in organic solvents like acetonitrile or dichloromethane works well, but it hardly budges in water thanks to the non-polar hexafluorophosphate.
Technical Specifications & Labeling
Suppliers offer the compound in varying purities, though anything below 98% usually isn’t much use in research. Labels on bottles typically note hazards—corrosive, causes severe eye damage, handle carefully. You’ll see recommended storage at room temperature, away from moisture, because the hexafluorophosphate anion can break down and release toxic hydrogen fluoride if water creeps in. Container types range from glass to plastic, and manufacturers sometimes add small drying agents to keep product quality stable.
Preparation Method
In my experience, making this compound in the lab took a few steps and some patience. The typical approach involves reacting 1,3-dimethylimidazolium chloride with potassium hexafluorophosphate in a polar organic solvent, such as acetonitrile. The reaction trades the chloride out for hexafluorophosphate, creating potassium chloride as a byproduct, which gets filtered out. The solvent usually gets removed under reduced pressure, and then you dry the product under vacuum to chase off trace water and solvents. Commercial production leans on this method, scaling up with specialized reactors and sometimes a column to purify the final product.
Chemical Reactions & Modifications
The compound resists most reactions at moderate conditions, though strong nucleophiles and reducing agents can snap the hexafluorophosphate ring apart, especially in the presence of water. Chemists sometimes use this property intentionally, for instance, to tweak electrolyte compositions in batteries or to generate fluoride ions under controlled breakdown. Derivatization at the imidazolium ring opens avenues for tailoring physical properties—sticking longer alkyl chains on the ring tips shifts solubility or viscosity, which changes how the liquid acts in separating mixtures or facilitating catalytic cycles. Reactions targeting the anion can swap out the hexafluorophosphate for other counterions, giving new ionic liquids with different thermal or chemical behaviors.
Synonyms & Product Names
This compound shows up under quite a few names. N,N-Dimethylimidazolium hexafluorophosphate, 1,3-Dimethylimidazolium PF6, and DMIM PF6 all point to the same salt. Some catalogs refer to it as a methylated imidazolium hexafluorophosphate ionic liquid. You find both the full chemical and shortened names in papers, which can frustrate folks searching for safety data or chemical literature. Consistency in product naming is something chemists, especially those new to the material, find valuable for reducing confusion.
Safety & Operational Standards
My early encounters with this compound reminded me how important it is to respect the hidden hazards of supposedly benign salts. The hexafluorophosphate ion poses risks because it can hydrolyze to form hydrofluoric acid, which attacks skin and glass. Eye and skin contact need serious prevention—goggles, gloves, solid protocols. Adequate ventilation is a must, since hydrogen fluoride gas lingers undetected until irritation or burns develop. Labs often keep calcium gluconate gel on hand for emergency treatment. Handling guidelines have improved after a few widely published accidents; now most chemical suppliers include detailed material safety data sheets, and industrial sites often double up on containment to prevent accidental releases. Training and respect for the material rank higher than ever in day-to-day operations.
Application Area
Use of 1,3-dimethylimidazolium hexafluorophosphate stretches pretty widely. Electrochemistry circles see this ionic liquid as a solid pick for solvent systems in supercapacitors or lithium-ion batteries. It doesn’t conduct electricity as well as water, but it brings better voltage windows and stability—important for long-lasting, powerful devices. Organic chemists lean on it as a reaction solvent for certain tricky syntheses where regular solvents would catch fire or decompose. Catalysis research loves it, especially for its ability to stabilize oddball intermediates or metal complexes. Extraction processes in analytical chemistry also harness this liquid’s taste for separating hydrophobic analytes. A few green chemistry protocols even point to this salt as a non-volatile alternative in pilot plants, though its cost and waste handling slow wider adoption.
Research & Development
In the last ten years, laboratories have shown a real uptick in publications on ionic liquids, and this hexafluorophosphate salt gets frequent mentions. Teams look at it for new battery electrolytes, focusing on safety and lifespan. Environmental chemists check recycling strategies, since ionic liquids bring cleanup challenges after use—nobody wants to trade one environmental mess for another. Synthesis research explores new catalysts dissolved in imidazolium salts, hoping to cut energy input or boost product yield. Developments aim for cheaper, less toxic versions, with alternative anions or improved capacity for recyclable use. With EU and US regulatory pushes for safer chemicals, R&D groups explore tweaks to molecular structure to balance technical gains against environmental impact.
Toxicity Research
Toxicologists dug into imidazolium-based liquids after early evidence showed not everything “green” proved safe for workers or waterways. 1,3-Dimethylimidazolium hexafluorophosphate scores low on volatility, meaning inhalation risk is less compared to traditional solvents. On the downside, ingestion and prolonged skin contact have led to tissue damage in animal studies. Breakdown with water yields hydrofluoric acid, raising alarm for facilities with poor containment. Regulatory agencies in Europe pushed for stricter labeling once environmental persistence and bioaccumulation became concerns. Many toxicology papers now call for closed-loop recycling, strict disposal, and careful handling, especially in labs with less experience dealing with hexafluorophosphate compounds.
Future Prospects
Innovation is pushing this area forward, but the biggest hurdles stick around cost, safety, and end-of-life disposal. Some researchers look to swap out the hexafluorophosphate anion for less hazardous alternatives, banking on similar physical benefits without the toxic byproducts. Battery developers test entirely new salts for better performance, but imidazolium hexafluorophosphate keeps its grip as a benchmark. Adoption might grow if operators can bring down production costs and close technical gaps on recycling and reuse. Regulatory pressure likely means industries take a tougher look at risk and reward. As energy storage, green chemistry, and analytical methods lean farther into ionic liquid territory, improvements in safety protocols and scalable recovery methods will mean as much as the material’s core technical bellwether qualities.
The Place of Ionic Liquids in Modern Chemistry
Ionic liquids have changed approaches to chemistry over the last two decades. Lab coats see them as game-changers due to their unique physical properties. Among these, 1,3-Dimethylimidazolium hexafluorophosphate stands out. You spot it in research papers on novel solvents, green chemistry applications, and energy storage. After using it in my own experiments, I can speak to its versatility and why researchers gravitate toward it.
Why Chemists Like This Compound
This salt melts at fairly low temperatures, which leads to a liquid nature over a wide range. Many research teams have ditched classic organic solvents in favor of this compound because it handles things traditional solvents just can’t manage. I remember dissolving tough organometallic complexes without the headaches from toxic vapors. The air in the lab felt cleaner, the sense of risk lower.
1,3-Dimethylimidazolium hexafluorophosphate is known for balancing strong solubility with low volatility. Handling it, I never worried about inhaling harmful fumes—something every chemist values after years around flammable solvents. Studies from the American Chemical Society back this up, highlighting its reduced environmental footprint compared to volatile organic compounds.
Practical Uses in the Real World
Researchers and industry use this ionic liquid as a solvent for a spectrum of chemical transformations. I’ve watched teams produce fine chemicals, catalysts, and pharmaceuticals using fewer purification steps thanks to its selectivity. Since it stays stable and doesn’t fly off into the atmosphere, it allows for easier recovery and reuse. In industry, this means less waste and lower costs—a clear win for both bottom lines and the planet.
In batteries and supercapacitors, you see even more promise. The compound’s ability to carry ions efficiently boosts the performance of devices that store and deliver energy. My friends in electrochemistry have been excited by the prospects for safer, more robust electrolytes in lithium-ion batteries.
You also find 1,3-Dimethylimidazolium hexafluorophosphate used in metal plating and extraction. In processes like these, its low reactivity with metals and predictable ionic behavior make it reliable. Extraction specialists can target precious metals from complex mixtures more effectively. The increased selectivity cuts down on contamination, an outcome any environmental scientist supports.
Looking at the Risks and Charting a Path Forward
Using new materials always brings questions about risk. Some ionic liquids raise eyebrows over toxicity to aquatic life. While researchers give this compound higher marks than older industrial solvents, studies have raised concerns about hexafluorophosphate’s environmental persistence. As a chemist, I have seen labs adopt rigorous containment and disposal procedures. Moving forward, combining advances in design with responsible use will matter.
Regulatory bodies and research councils have started pushing for more biodegradable, less persistent alternatives. Professional societies share open data on toxicity, guiding safer handling and disposal. Encouraging companies to invest in recycling technologies and closed-loop systems will help ensure benefits don’t come at a hidden cost.
Building a Responsible Future in Chemistry
Every tool comes with pros and cons, but the use of 1,3-Dimethylimidazolium hexafluorophosphate marks a move toward greener practices. By weighing safety data and investing in new recycling strategies, chemistry can keep pushing boundaries without leaving a toxic legacy behind. Through my own hands-on work with this compound, I’ve witnessed both its transformative power and the care required to handle it responsibly.
Assessing the Hazards
I once watched a colleague clean up a minor spill of an ionic liquid, confident that a lab coat and gloves would be enough. It turned out the fumes irritated his throat for hours, and he spent the rest of the day coughing. That experience solidified the need for more caution with chemicals like 1,3-Dimethylimidazolium hexafluorophosphate. This compound, often used as a solvent or electrolyte, comes with clear dangers. Touch or inhale too much and you risk skin burns, eye damage, or even respiratory distress. A safety datasheet tells the story plainly, but seeing reactions up close drives the point home more than any printout.
Personal Protective Equipment Really Matters
A basic lab coat won’t cut it. I’ve found that nitrile gloves hold up well against most solvents, but for this compound, double-gloving with nitrile offers extra peace of mind. Standard goggles protect eyes, but full-seal safety goggles stop vapors from sneaking in. Chemical splash face shields add a further layer of defense. Fumes demand a respirator—never rely on room ventilation alone. I once worked in a lab where a barely-there whiff of solvent went unnoticed until someone felt dizzy. Playing it safe prevents long-term harm.
Ventilation Outweighs Comfort
Good ventilation makes all the difference. Fume hoods are built for jobs like these—they pull dangerous vapors away, keeping everyone out of the danger zone. Years ago, I worked at a bench without proper airflow. After some hours with open bottles, headaches and a lingering, metallic taste told me something was wrong. Now I always double-check that the sash is down and airflow is strong before opening any bottle. Even small exposures add up over days or weeks. Labs with proper airflow systems keep workers healthy, plain and simple.
Storage and Spill Response
Working with this compound is more than just personal protection. Sealed, labeled containers reduce the chance of accidental exposure. I keep incompatible reagents apart because chemicals never mix well with surprises. For clean-up, absorbent pads, neutralizing agents, and dedicated waste bins belong within arm’s reach. Years back, a beaker slipped and shattered—quick cleanup kept the problem from spreading. It’s never about bravado; it’s about protecting everyone in that room.
Training Isn’t Optional
I used to think reading the datasheet once a year covered everything. Real safety depends on hands-on, repeated training and drills. Knowing what to do, even with shaky nerves, beats relying on memory or guessing. New team members often have loads of questions—encourage them to speak up. In my experience, complacency causes accidents. Sharing stories and reviewing protocols keeps everyone sharp and aware.
Solutions Are On-Going
Chemical research keeps evolving, safety practices have to keep up. Regular inspections catch old gloves or faulty fume hoods before they cause problems. Working in teams, sharing near misses without blame, and keeping communication open forms the backbone of lab safety. The habits we form—checking labels twice, wearing all the gear, respecting warning signs—protect more than just our research. They safeguard each person who steps foot in that space.
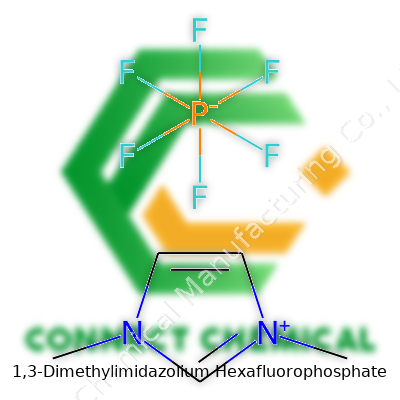
The Molecular Formula and Structure
1,3-Dimethylimidazolium hexafluorophosphate sits in a unique spot among ionic liquids, built from two main parts: the cation 1,3-dimethylimidazolium and the anion hexafluorophosphate. The molecular formula tallies up to C5H10N2·PF6. Anyone familiar with chemistry labs will recognize imidazolium-based salts by their distinctive structure—a five-membered imidazole ring with nitrogen atoms at positions one and three, both of which bear methyl groups. The anion never tries to steal the limelight; PF6− comes together in an octahedral arrangement, surrounding phosphorus with six fluorine atoms.
Real Impact in the Laboratory
I recall the first time I handled a small vial of this salt. It looked innocent enough, colorless and crystalline, but the moment you introduced it to the right solvent, it told a different story—a viscous fluid emerged, nothing like traditional organic solvents. This behavior underpins why chemists across the globe reach for it. Its low volatility means fumes never chase you from the bench. Electrochemists appreciate its ability to dissolve a variety of metal salts—a feature particularly handy in nonaqueous electrochemical cells and battery research.
Why Structure Matters
One small twist in a molecule's structure can make a massive change in its properties. The methyl groups at positions one and three on the imidazolium ring aren't just window dressing. They boost stability and limit side reactions, making this compound less reactive than analogs with hydrogen at those positions. The tight ion pairing between imidazolium and hexafluorophosphate dictates how it dissolves in water or organic solvents, how it carries charge, and even how it interacts with pharmaceuticals or catalysts. With C5H10N2+ sitting on top of PF6−, the overall neutral salt avoids the problems typical for volatile and often flammable organic solvents.
Supporting Facts from Research
Papers published in Green Chemistry and the Journal of the American Chemical Society show that 1,3-dimethylimidazolium hexafluorophosphate has carved out a niche in sustainable chemistry. Its ability to dissolve cellulose got plenty of people excited. According to a 2022 review, ionic liquids cut down waste and energy costs when compared to traditional solvents that rely on harsh acids.
Tackling Real Issues in Use and Disposal
You won't find a chemical compound without its headaches. The stability of the PF6− anion brings up environmental concerns. It can break down into harmful byproducts, like hydrofluoric acid or phosphorus oxyfluoride, especially with improper disposal or in the presence of water and heat. Researchers at several European universities have pointed this out for almost a decade. Labs started looking for alternatives, such as using less toxic and more biodegradable anions—acetate or other weakly coordinating species.
Solutions and Safer Handling
Handling starts with respect for what the material can do and the risks involved. Companies and academic labs shifted to tighter controls—gloveboxes, proper ventilation, and detailed disposal instructions printed on every bottle. Some are seeking new imidazolium salts that trade out PF6− for greener anions. Another strong strategy involves recycling protocols, where used ionic liquid gets cleaned up and reused, cutting waste and saving money.
Looking Ahead
Innovation isn't magic; it often springs from gradual tweaks and honest looks at the bigger picture. 1,3-Dimethylimidazolium hexafluorophosphate still finds a spot on many laboratory shelves. Its precise structure and predictable properties help drive progress in diverse fields, but the pressure to improve safety and sustainability keeps pushing chemists to redesign the formula just a little better each time.
Why Storage Matters
Storing chemicals like 1,3-Dimethylimidazolium Hexafluorophosphate isn’t just about ticking off a checklist. With ionic liquids, the dangers are real and so are the consequences if somebody cuts corners. Chemical spills or accidental releases have a way of showing up in headlines. As someone working with research teams in academic labs, I’ve seen how a single overlooked cabinet lock or wrong room temperature can turn into bigger safety conversations that no one wants to have.
Practical Steps for Storage
1,3-Dimethylimidazolium Hexafluorophosphate doesn’t play well with water or atmospheric moisture. Leave a bottle open, and suddenly you’re dealing with a chemical that’s not as pure as you thought it was. I’ve watched lab techs scramble, trying to explain away decomposed samples because a container was only “slightly” loose.
I keep this ionic liquid sealed tightly—airtight containers make a real difference. Glass works well, but not every plastic holds up against fluorinated compounds. I prefer bottles with PTFE-lined caps because I’ve seen standard plastic degrade far too quickly. Tucking these containers away from sunlight helps too, since UV exposure speeds up unwanted reactions. On more than one occasion, I’ve had to remind team members that a dark cabinet isn’t optional. It makes or breaks sample integrity.
Temperature comes up often in safety meetings. Those storing 1,3-Dimethylimidazolium Hexafluorophosphate at room temperature usually do fine, but sudden spikes or drops bring risk. Once, we lost a batch because the heating system failed and condensation slipped into a jar. So I keep stocks at a steady, dry, cool spot—never near radiators or the back of fume hoods where temperatures swing daily.
Personal Protective Equipment and Location
Every time I open a container, I put on gloves and eye protection. The hexafluorophosphate ion doesn’t mix well with human health; skin contact brings its own set of worries. Storing the chemical on a low shelf reduces the chances of falling. I remember someone placing heavy chemical bottles up high to save bench space, only to knock one off—luckily it was empty, but the lesson stuck.
More experienced lab managers keep a clear record of what’s inside each bottle. Labeling sounds simple but really pays off. Sharpie on tape doesn’t cut it—over time the ink fades. I prefer indelible markers or printed labels with the full chemical name and hazard warnings. During an unexpected inspection, those details protect everyone in the room.
Disposal and Emergency Responses
If a spill happens, panicking wastes precious seconds. Absorbent pads and neutralizers need to sit close by. It’s no use storing safety supplies in a different building or down two flights of stairs; accidents grow quickly. I always talk my team through the exact steps—cover the spill, isolate the area, and follow up with ventilation.
Proper disposal matters just as much as storage. I never pour leftover 1,3-Dimethylimidazolium Hexafluorophosphate down a drain. The local hazardous waste program wants a full log, so I document every bit. In my experience, working openly with regulators keeps everyone’s mind at ease and builds trust in the lab’s professionalism.
Smart Habits Build Safer Labs
Solid storage habits don’t just lower risk—they help everyone sleep a little better. Labeling, using the right containers, and keeping supplies ready save more than just time. For labs and workplaces handling 1,3-Dimethylimidazolium Hexafluorophosphate, making safety second nature protects people and keeps science moving forward.
What’s in a Solvent?
Ask anyone who’s spent time in a lab: knowing what dissolves in what can save hours of frustration. 1,3-Dimethylimidazolium hexafluorophosphate, or [dmim][PF6], finds itself right in the middle of this classic chemistry question. You’ve got a liquid salt with a bulky imidazolium ring and a hefty PF6 anion. Most people expect ionic liquids to blend seamlessly with water—but this one plays by different rules.
Water or Not?
I remember an old rule from my college days—“like dissolves like.” In real life, this rule stumbles, especially with salts like [dmim][PF6]. Water doesn’t accept it well. You drop some into a beaker and watch the crystals float around, undisturbed; add a stir bar and nothing much happens. The hexafluorophosphate anion resists water, almost stubbornly, thanks to fluorine’s tight grip and the overall low polarity. If you ever tried dissolving it cold, you’d probably wonder if you grabbed the wrong container entirely. So, no, don’t rely on water to do the heavy lifting if you’re dealing with this salt.
Organic Solvents: A Better Fit
Turn instead to organic solvents. Acetonitrile, dichloromethane, even acetone—these get the job done. In my experience, acetonitrile strikes a sweet spot: it’s polar enough to break apart the ions without stealing away protons or reacting unpredictably. Ethanol gives mixed results, tending toward cloudiness and incomplete dissolution. Solubility charts back this up, reporting high solubility for [dmim][PF6] in acetonitrile, moderate in DMSO, and minimal in hexane or nonpolar options.
Why Solubility Choices Matter
This isn’t just about seeing a clear solution. Labs depend on effective solvents to run reactions cleanly, recycle products, and keep safety hazards low. Picking the wrong solvent wastes time and shoots up costs. My own misstep involved using ethanol as a shortcut, only to waste an afternoon filtering and redissolving. Productivity drops quickly if you’re always retrying dissolutions. Plus, using the right solvent means less chance of side reactions or unwanted byproducts—a huge issue in pharmaceutical and battery research, where this salt often appears.
Safety Considerations and Green Chemistry
Choosing safer solvents is more than a trend or green-washing. Toxic chlorinated solvents come with disposal headaches and fumes nobody wants to breathe. Acetonitrile isn’t perfect, but it beats dichloromethane on most safety metrics. In future projects, colleagues have switched to methyl ethyl ketone or even supercritical CO2, putting health and sustainability a little higher on the list. New research into biodegradable or water-compatible salts might even make the whole question a thing of the past someday.
Solutions and Smarter Choices
Planning is easier with tested solubility data. Before mixing anything in the flask, it pays to check manufacturer data sheets, talk to labmates with experience, and run quick small-scale tests. If water is absolutely necessary, consider other ionic liquids designed to play well with it. For now, acetonitrile holds the throne for dissolving 1,3-dimethylimidazolium hexafluorophosphate quickly, efficiently, and with fewer regrets down the line. Being practical about solvent selection doesn’t just protect the science, it makes the whole process smoother and more predictable—something every chemist appreciates.