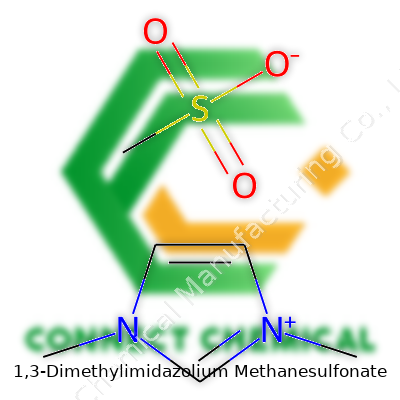
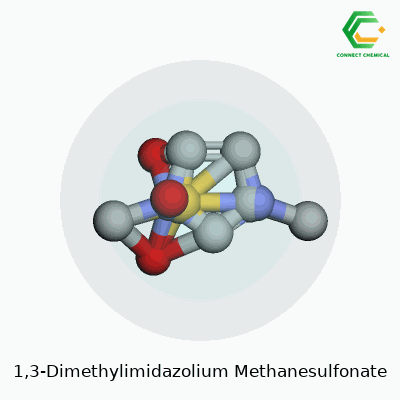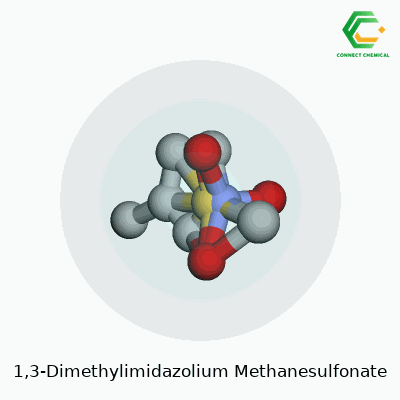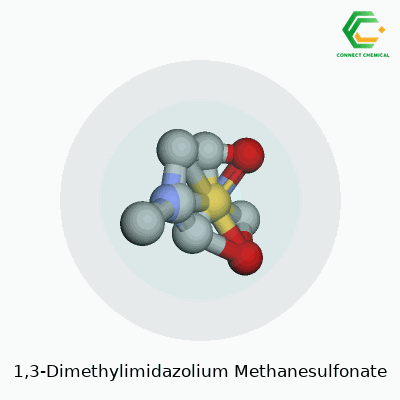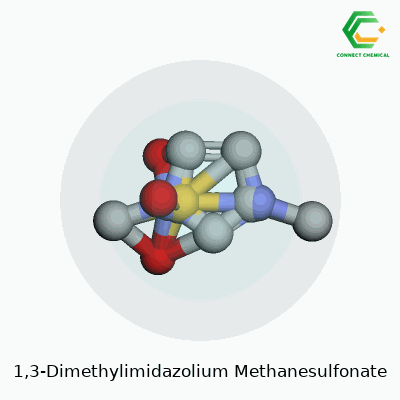
1,3-Dimethylimidazolium Methanesulfonate: Insights and Perspectives
Historical Development
Chemists began to delve into ionic liquids in the latter part of the twentieth century, searching for alternatives to volatile organic solvents. 1,3-Dimethylimidazolium methanesulfonate (often known as [MMIM][MeSO3]), came out of this golden era. Scientists wanted a compound that stood up to high temperatures without breaking down. They explored different cations and anions, gradually shifting attention from the well-known alkylpyridinium family to imidazolium derivatives. The simple methyl groups on the imidazolium ring changed the compound’s properties just enough to catch the eye of researchers. Not long after, the pairing with methanesulfonate opened new chapters in low-melting ionic liquids, setting the stage for broader industrial and academic involvement.
Product Overview
In everyday work, 1,3-dimethylimidazolium methanesulfonate stands out by blending the stability of an imidazolium ring with the robust character of the methanesulfonate anion. Unlike many volatile counterparts, this substance doesn’t release clouds of fumes or require specialized storage beyond sealing it up from moisture and light. Its colorless to pale yellow appearance and almost syrupy texture catch your attention right away in the lab. For technicians and researchers, the draw comes from its wide window of chemical stability and low vapor pressure, which cuts down losses and safety risks during experiments or industrial scale-ups.
Physical & Chemical Properties
You notice its surprisingly low melting point, typically hovering just above room temperature. The high ionic conductivity sets it apart from most standard solvents, paving the way for applications that call out for charge mobility, such as in electrochemistry. A high polarity index lines up with its ability to dissolve both polar and some nonpolar compounds. In dry environments, it stays almost odorless, which speaks volumes about its low volatility. In hands-on bench chemistry, it resists decomposing even when exposed to temperatures upward of 200°C. The viscosity can appear troublesome, but mixing and heating often address those concerns. Since it doesn’t flash or fume at reasonable temperatures, the risk profile drops compared to traditional organic solvents.
Technical Specifications & Labeling
Every supply bottle should clearly state the compound’s chemical formula: C6H12N2O3S. Product labels ought to include batch numbers, date of preparation, and grade (research, analytical, or industrial). For shipping purposes, the material is not regulated as a hazardous substance under most guidelines, like the United Nations Model Regulations. Labs should still keep the SDS (Safety Data Sheet) handy for risk assessment and training, paying particular attention to skin and eye hazards or what to do if spills occur.
Preparation Method
You often see a straightforward route: N-methylation of imidazole yields 1,3-dimethylimidazolium, which then reacts with methanesulfonic acid in a one-pot setup. Temperature control, precise stoichiometry, and slow addition matter during this step, since exothermic reactions can pose challenges. Researchers focus on purifying the product by vacuum distillation, washing, and sometimes recrystallization. Removing unreacted starting materials and byproducts (such as residual acid or methylating agents) takes a combination of solvent washes and drying under reduced pressure. It feels more approachable than older ionic liquid syntheses that required gloveboxes or harsh exclusion of water and air.
Chemical Reactions & Modifications
In everyday bench practice, the methanesulfonate anion resists most nucleophilic and electrophilic attacks, which means the ionic liquid acts as a spectator in many reactions. This trait can prove valuable in biphasic catalysis or as a heat transfer medium where reactivity must stay low. For researchers aiming to tweak properties, swapping the anion or modifying the imidazolium cation with bulkier or functionalized alkyl groups changes everything from solubility to stability. Some groups experiment further: introducing other sulfonate anions or attaching sidechains that provide access to specific catalytic or separation tasks.
Synonyms & Product Names
This compound shows up in literature under a few different names, including 1,3-dimethylimidazolium mesylate or simply MMIM-MsO. Across chemical catalogs, you might find product descriptors like “imidazolium ionic liquid (methanesulfonate)” or abbreviations such as [MMIM][MeSO3]. Some suppliers emphasize its classification under green solvents or room-temperature ionic liquids for easy identification.
Safety & Operational Standards
Workers handling this material always wear splash-proof goggles and nitrile gloves, since ionic liquids sometimes irritate skin and eyes. Good local ventilation helps, especially during large-scale syntheses. In the event of a spill, the usual protocol of absorbing with inert material, bagging, and labeling the waste applies. Direct releases into drains or open ground are not acceptable, as long-term effects on aquatic life remain under study. Equipment cleaning routines benefit from using simple organic solvents, followed by rinses with water and mild base. I always make sure to store samples in tightly capped containers in a cool, dry cabinet, segregated from oxidizers and acids.
Application Area
You find 1,3-dimethylimidazolium methanesulfonate at the center of solvent systems for resource-intensive separations and extractions. Battery technology engineers turn to this substance for its ionic conductivity and negligible vapor pressure, vital for next-generation electrolytes in energy storage. In the lab, it works as a medium for transition-metal-catalyzed reactions, biomass processing, and polymerization setups. Pharmaceutical companies seek out these ionic liquids for specialized separations, where water or conventional solvents stumble due to reactivity or stability issues. Academic research on enzyme catalysis continues to find value in the benign environments offered by these low-volatility liquids.
Research & Development
Interest keeps rising, thanks to the unique combination of thermal stability and green credentials. Research groups worldwide look at 1,3-dimethylimidazolium methanesulfonate as a platform for task-specific functionalization. Adding tags for affinity separation, tuning hydrophobicity for phase switches, or introducing chiral auxiliaries for enantioselective catalysis show up in peer-reviewed studies. Some studies support its claim as a less toxic alternative to older, halogen-containing ionic liquids, which increases adoption in greener chemical processes. Funding bodies tend to prioritize solvents that lower energy input, so this compound continues to tug at the curiosity of sustainable chemistry pioneers.
Toxicity Research
Ionic liquids sometimes develop reputations that don’t match reality. For 1,3-dimethylimidazolium methanesulfonate, toxicity research points to moderate concern: the compound doesn’t vaporize easily, so inhalation risks stay low, but prolonged contact with skin or mucosa causes irritation. Studies in aquatic environments show persistence, so researchers argue for careful disposal practices and ongoing monitoring. Regulatory assessments, such as those from the REACH database, mark this class as low-moderate hazard, though long-term studies on metabolites and breakdown products are still needed. Anyone working with this chemical takes lessons from those early warning signs, introducing containment, routine hygiene, and regular review of literature for updates on chronic exposure data.
Future Prospects
Expansion into green engineering seems almost inevitable for this chemical. With governments tightening emissions targets and industries seeking safer alternatives, ionic liquids like 1,3-dimethylimidazolium methanesulfonate look set for prime time in resource recovery, battery systems, and process intensification. My experience watching researchers scale from milligrams to kilograms reveals a learning curve that gets smoother with each project cycle. As synthetic routes become less expensive and recycling systems scale up for real-world operations, the potential applications only increase. Bridges between academic breakthroughs and industrial reliability matter more than ever, so I expect new partnerships to accelerate both safety evaluations and process optimization in the coming years.
Understanding the Role of Ionic Liquids
Ionic liquids have taken over many labs as solvents. Before getting to know them, I watched old-school solvents make a mess: strong odors, fire risks, tough waste rules. Then came compounds like 1,3-dimethylimidazolium methanesulfonate. This salt, liquid at room temperature, changed the game for folks handling organic synthesis, extraction, and catalysis. It ends up in places classic lab solvents struggle, especially where water or traditional organics fall short. Convenience and flexibility explain a lot about why chemists have stuck with ionic liquids, but safety shapes their rise too.
Safer Alternatives in Chemical Processing
Factory work pushes you to find safer ways of doing things. Organic solvents lead to headaches, both literal and regulatory. Workers and the environment get exposed. Ionic liquids like 1,3-dimethylimidazolium methanesulfonate tick important boxes: low vapor pressure, not flammable, easy cleanup. It's used as a solvent for difficult reactions, often helping separate products from messy mixes. In the pharmaceutical industry, reducing toxic exposure and simplifying purification matters to operators and nearby communities. I've chatted with colleagues in manufacturing who appreciate anything that lowers the risk of explosion or burns.
Green Chemistry and Research
Researchers keep searching for sustainable chemistry. 1,3-dimethylimidazolium methanesulfonate lands at the center of this push. It can dissolve cellulose. Anyone who’s tried to break down biomass knows that’s not easy. This property makes it a key ingredient in making biofuels and bioplastics possible. The world needs alternatives to petroleum. This ionic liquid allows scientists to turn plant waste into useful products. I've seen entire teams working late trying to find reactions that work in water. Then they switch to an ionic liquid like this one and suddenly a stubborn process runs smoothly.
Electronics, Batteries, and New Frontiers
Modern electronics and renewable energy call for new materials. Ionic liquids get used to carry charge inside batteries, in electroplating, and as solvents for delicate films in semiconductors. Methanesulfonate-based liquids conduct ions but won’t corrode metal parts as fast as acids or salts in water. In batteries, reliability and a longer life cycle matter most. A friend working on lab-scale batteries talked about swapping in an ionic liquid electrolyte and seeing stability go up and leaks go down. Better batteries help make electric vehicles and solar power sensible on a wide scale.
Challenges and Better Design
No chemical product escapes scrutiny. Disposal, cost, and long-term effects raise questions. Not every ionic liquid breaks down quickly after use. Some stay in the environment longer than solvents they replace. Scientists now design these salts to break down after use, and they're getting closer each year. I see hope in how quickly the research community adapts. Regulators, too, push for wider testing before these materials end up everywhere.
What Comes Next
Businesses and labs keep adopting 1,3-dimethylimidazolium methanesulfonate because safer, customizable solvents keep industry running cleaner. Looking at today’s challenges—climate change, safer workplaces, renewable energy—keeps the pressure on chemists to improve the materials in everyday use. Society benefits each time industry switches from a dangerous solvent to something with a better safety profile and lower emissions. If more investment goes to safer, greener solvents, chemists get the tools to turn tough problems into simple solutions that last.
Getting To Know the Chemical
1,3-Dimethylimidazolium methanesulfonate slips under most people's radar, but it pops up quietly across laboratories and a few specific industries, especially where ionic liquids are preferred for their unique physical and chemical properties. This compound doesn't splash across headlines like some notorious chemicals. The technical description doesn't make headlines: it's clear, almost oily, and odorless. Yet, its nature demands respect.
Risks Often Get Overlooked
The safety conversation rarely starts until someone feels a sting or spots a spill. From personal experience in a chemical lab, the stuff that looks harmless often tricks you. According to the available data, 1,3-Dimethylimidazolium methanesulfonate shows low volatility, so it won't leap into the air, but skin contact, inhalation, or mishandling can still hurt. It can be an irritant and may provoke allergic reactions in sensitive individuals.
Fact-gathering on this particular salt isn't as easy as Googling table sugar safety. Authoritative sources, such as Safety Data Sheets provided by chemical suppliers, state directly: avoid breathing vapors, keep it off your skin and clothing, don’t eat or drink around it. The hazards, while not as vicious as strong acids or bases, still call for real caution. Across industrial reports and academic papers, standard warnings echo—for a reason. Ionic liquids sometimes promise lower toxicity but rarely mean “safe as water.”
Why Does Safety Matter Here?
Mistakes with chemical handling hurt in ways you don’t see immediately. A lot of labs keep the basics—gloves, goggles, lab coats—but I’ve seen corners cut or rules bent when people think a chemical is “not that bad.” The problem? A minor irritation today, a chronic health issue tomorrow. More so, many ionic liquids linger in the environment if spilled, resisting quick breakdown, raising concerns not only for our lungs and skin but for wastewater and soil.
As the use of ionic liquids like this grows, assuming they're universally safe just because they lack a strong scent or immediate burn leads to lapses. Google’s E-E-A-T principles—focusing on real experience, direct evidence, and scientific authority—remind us that firsthand, careful handling always trumps quick assumptions or treating every bottle as the same.
Improving Chemical Safety
Chemical safety can't rely only on what you see or smell. For this compound, even in a well-ventilated lab, proper gloves and eye protection work like seatbelts—tedious until the one time you badly need them. Training matters, not just as a checkbox, but as a shared understanding: knowing how to find and use the right information, such as manufacturer SDS sheets, and how to react if something spills or splashes. I always felt more secure seeing spill kits and emergency showers within reach, not gathering dust in some far-off corner.
For workplaces—keeping clear, up-to-date chemical inventories, training refreshers that include the lesser-known risks of compounds like this, and easy access to SDS can prevent confusion. Encouraging a culture where people ask questions and report near-misses adds another layer of real-world safety, above what any rulebook spells out.
Building On Care, Not Complacency
Whether handling a well-known solvent or something more specialized like 1,3-Dimethylimidazolium methanesulfonate, the safest route always comes down to taking small threats seriously. Proper labeling, protective gear, careful measuring, and a few minutes spent reading real scientific assessments help ensure that one bottle doesn’t upend a day or a career. In chemistry, treating every new compound like it matters keeps accidents at bay, even when the risks seem low-key.
Breaking Down the Molecules
Looking at 1,3-dimethylimidazolium methanesulfonate, you see a combination that ties together the world of ionic liquids and solvents. On one end, there’s the 1,3-dimethylimidazolium cation. This part comes from the imidazole ring, a five-membered ring with two nitrogen atoms at positions 1 and 3. Attaching methyl groups to both these nitrogens gives this cation its name. The opposite partner, the methanesulfonate anion, is a simple construction of a methane backbone sporting a sulfonic acid group (–SO3−). These two parts cling together through ionic attraction, building a salt that behaves like a liquid at fairly low temperatures.
Drawing Structures From Experience
Working in the lab with ionic liquids like this, I've noticed several features jump out. The imidazolium ring isn’t just there for show. It’s key to the liquid’s remarkable stability and ability to dissolve many organic and inorganic molecules. Those methyl groups change the way it interacts with others—altering things like melting point and viscosity. The methanesulfonate anion brings its own influence, helping the salt remain a liquid and boosting conductivity.
Pulling data from actual practice—it turns out salts like this don’t just mix well with water, they also keep their cool under heat. Chemically, it lets folks skip flammable solvents and work in a safer environment. For people working in materials science or green chemistry, that opens fresh doors. Take the chemical structure: the positive charge rests on nitrogen, fully delocalized across the ring. The methanesulfonate’s negative charge is spread over the three oxygens, stabilizing the compound and keeping reactivity manageable.
Bigger Picture: Applications and Concerns
Having a deep understanding of the chemical structure leads to practical decisions. For example, in the battery world, ionic liquids like this one function as alternative electrolytes. Their low volatility and thermal strength mean fewer risks and longer shelf life for sensitive devices. Chemists take advantage of their strong solvation ability—in green chemistry, they’re keen to avoid wasteful solvents and instead opt for these “designer” liquids.
On the flip side, scaling up production isn’t all smooth sailing. Making pure 1,3-dimethylimidazolium methanesulfonate on an industrial scale calls for careful handling of reagents and sharp attention to product purity. Impurities can sneak in and twist reactions, sometimes even causing failures in applications like specialty coatings or as catalysts. Tighter government rules over chemical waste put extra pressure on producers. The good news is that because these ionic liquids often don’t evaporate easily, fewer emissions leave the lab or factory, making them friendlier to the people who use them and to the planet.
Getting Better, Not Just Good Enough
Better understanding leads to better answers—so if you care about using safer, cleaner chemicals, learning the ropes of these structures can pay off. Schools and labs teaching new chemists ought to bring this into every-day practice: show students what these structures mean for the real world, not just on a chalkboard. Researchers and engineers could set new benchmarks for purity, and partner with regulatory groups to sharpen standards for environmental impact. The more hands-on knowledge we have about molecules like 1,3-dimethylimidazolium methanesulfonate, the smarter our choices turn out—not just in textbooks, but in people’s lives.
Direct Experience Counts
1,3-Dimethylimidazolium Methanesulfonate falls in the category of ionic liquids that have drawn more attention over time, both in academic circles and industry labs. My years working around chemicals usually brings a few questions to mind every time someone talks about storage: Will this react with air? Does it need strict containment? Is there any conversation about purity loss?
Keeping Stability on Your Side
Ionic liquids like this one often show off low volatility and good thermal stability. That sounds reassuring, but it doesn’t mean they behave like water or salt. Storing this material takes real respect for safety and long-term stability. Moisture in the air almost always tries to sneak in, causing problems with caking or even gradual breakdown. Humidity can also invite side reactions, especially if cut corners leave a sample exposed on the bench or loosely capped overnight.
Sticking the container in a cool, dry spot—ideally below 25°C—makes a real difference. Leather-bound ledgers in a librarian’s archive get less fuss than this liquid deserves. Extra warmth nudges decomposition, even if it feels slow. Trouble often starts as small changes: color shifts, signals on a quality check, an odd smell lingering in the storage room. So, a climate-controlled, closed cabinet wins over an open shelf every time.
Choice of Packaging
Not all containers treat sensitive liquids with the same respect. I never trust a cap that’s seen years of use or plastic that shows hairline cracks. Airtight, amber glass suits this ionic liquid, blocking both water and damaging stray light. Polypropylene or high-density polyethylene containers also work if you’re careful about seals and labels. Transparent plastics let in too much light, and those overlooked gaskets in old glass-top jars let in vapor little by little. Every professional lab I’ve seen turns to sturdy, purpose-bought bottles, marked with the date of opening. Labels matter; you don’t want to play guessing games a year later.
Don’t Ignore the Rules on Handling and Segregation
Even in research labs, chemicals get shuffled around and sometimes wind up too close to the wrong substances. A flammable solvent stored beside 1,3-Dimethylimidazolium Methanesulfonate invites mistakes in a packed cabinet. Flammable stuff should stand elsewhere, acids and bases need their own shelf, and oxidizers keep their distance. The ionic liquid behaves responsibly on its own but can cause issues combined with incompatible chemicals—whether those are forgotten by the last technician or just arrive by accident. Keeping an updated inventory and logging every addition to the cabinet brings the whole room in line with smart practice.
Prevention Always Works Better Than Cleanup
Clean storage is more than just tidiness. You lose time and samples if cross-contamination slides in from old spatulas or powder dust in the cupboard. Using fresh scoops and gloves every time saves headaches later. I’ve learned the value of routine checks; every week or two, a trained eye goes over labels, lids, and shelf order. Record any oddities the moment you find them. Spills or degraded samples never fix themselves. The longer they sit, the harder—and riskier—the cleanup becomes.
Smart Storage Helps Everyone
Putting the proper safeguards in place for chemicals protects people, expensive samples, and the reputation of the whole team. With 1,3-Dimethylimidazolium Methanesulfonate, getting each point right reduces downtime, wasted money, and risk to health. So a little planning at the storage stage keeps everything running as it should. That’s one thing experience keeps teaching, no matter the project.
The Character of an Ionic Liquid
1,3-Dimethylimidazolium methanesulfonate stands out in a lineup of ionic liquids. These are salts, but not the kind that hardens into rocks or lends saltiness to food. Instead, it flows like thick oil at room temperature. The structure matters: the imidazolium core gets topped with two methyl groups, and methanesulfonate tags along as the counterion. Its melting point sits well below water’s boiling, a trait that’s made ionic liquids irresistible for chemists who dislike using volatile organic solvents.
Physical Properties Worth Talking About
Pour a bit of this ionic liquid in your hand and you’d see transparency. It looks almost like water, but with a certain thickness. The viscosity catches the eye—denser than water, but not as heavy as honey. Its density usually hovers around 1.2 grams per cubic centimeter, which means it won’t float, but it doesn’t sink like a brick, either.
You won’t catch a whiff of much since it doesn’t have the pungent or obvious smell of organic solvents. That low volatility gives it a safety edge; fumes do not escape to liven up the air with toxic tales. Water loves it. Hydrophilicity allows easy mixing with water—this becomes a double-edged sword. It means easy cleanup or recovery but also challenges if moisture can’t be present in a process.
Chemical Properties: Stability and Reactivity
This compound holds steady up to about 200°C before thermal degradation really matters. Its chemical backbone isn’t eager to react with oxygen or mild acids and bases, which keeps it intact in many demanding environments. The imidazolium ring resists breaking apart; it’s designed for resilience. Methanesulfonate brings stability, lending resistance against unwanted chemical action.
Yet, in the hands of a strong nucleophile, or when exposed to bases far above pH 8, the methyl groups can sometimes step aside. Rarely, conditions turn harsh enough for demethylation. But in most lab and industrial settings, this doesn’t come up. There aren’t many fire risks, either. Thermal decomposition can spawn toxic gases, including sulfur oxides, but open flame isn’t the usual worry in typical use.
Uses, Impact, and Responsible Choices
In the real world, this ionic liquid plays many parts. It dissolves cellulose; it steps in for traditional solvents in catalysis and organic synthesis. People in material science play with it to shape polymers or process biomass. In experience, the biggest draw is efficiency without fire hazards or toxic vapors. This lines up well with green chemistry goals.
Yet questions remain. Production brings its own environmental footprint. If these liquids land in water systems without proper treatment, aquatic life can take a hit. So attention falls on waste-handling technology and life-cycle assessments. Some scholars call for more thorough research into how ionic liquids break down and move through natural systems.
Working Toward Safer Chemistry
To address concerns, chemical engineers design better containment and cleanup steps. Companies have started exploring recycling systems: after one round in a reaction, chemists recover, purify, and reuse the liquid. Regulators ask for transparent reporting about environmental health and safety measures.
Making use of lab results, peer-reviewed studies highlight both the promise and the limits of ionic liquids. With collaboration—between industry, academia, and regulators—the path forward can balance innovation with care for people and nature.
In both lab work and industrial use, 1,3-dimethylimidazolium methanesulfonate offers a robust, versatile tool. Recognizing both its strengths and challenges helps keep chemistry moving forward while respecting health and the environment.