1,3-Dimethylimidazolium Propane Bromide: A Close Look at an Ionic Liquid Shaping Modern Chemistry
Historical Development
Walk into any laboratory dealing with ionic liquids and you’ll probably hear the story of how chemists searched for alternatives to volatile organic solvents, chasing something stable, green, and useful. The imidazolium family answered the call. The introduction of a methyl group at the 1 and 3 positions on the imidazole ring changed the properties and pushed these salts from curiosity into the spotlight. Building off that, connecting a propane chain and adding a bromide gave us 1,3-Dimethylimidazolium Propane Bromide. Over the last two decades, researchers started applying it across the domains—synthesis, materials, and electrochemistry—showing what’s possible with a simple tweak to molecular architecture.
Product Overview
1,3-Dimethylimidazolium Propane Bromide comes in as a pale, usually hygroscopic solid, attracting water as soon as it hits laboratory air. With its ionic nature, it moves between roles—sometimes a solvent, sometimes a reactant, sometimes a medium for catalysis. Producers usually target high-purity grades, especially for electrochemistry, because even trace metal or halide contamination can swing outcomes in unexpected ways. Labs use it for handling cellulose, solvents for difficult separations, and as a reaction medium where typical organics fail. What sets it apart is a toolbox of features: strong ion exchange, thermal stability, and nontoxicity compared to legacy solvents.
Physical & Chemical Properties
An eye test in the vial shows a white to slightly off-white powder. It dissolves easily in water and polar solvents, showing why it fits into both wet and dry laboratory setups. The melting point usually sits around 80°C, but it shifts depending on batch, humidity, and sample prep. That low melting point means it nudges into 'liquid' territory for many applications, unlike table salt or other traditional ionic solids. The bromide anion tips the balance between hydrophilicity and hydrophobicity, making it ideal for phase transfer and cross-phase reactions. The compound delivers decent thermal stability up to 250°C, past which decomposition sets in and bromide release becomes a headache. In NMR and IR, its signature is unmistakable, letting operators check identity and purity without fuss.
Technical Specifications & Labeling
Labs and manufacturers focus on detailed technical pointers for this compound. Purity marks range up to 99%, and moisture content stays below 1% by Karl Fischer titration. Reliable suppliers provide certifications for trace metals, halides, and other potential interferences, all backed by analytical HPLC or GC. Labels list batch number, packing date, and hazard codes—specifically water reactivity and possible irritancy. Researchers run checks for melting point and elemental composition (C, H, N, and Br), confirming each bottle before use. Chemical manufacturers also issue shelf-life projections, which tends to run two to three years if stored dry at room temperature away from light.
Preparation Method
Preparative work on 1,3-Dimethylimidazolium Propane Bromide usually takes two core steps. Synthetic chemists start with 1,3-dimethylimidazole, brought into contact with 1,3-dibromopropane in anhydrous acetonitrile or another polar aprotic solvent. The reaction proceeds via an SN2 displacement, making the bond between the imidazolium core and the propane linker while delivering the bromide as counter-ion. Post reaction, the crude mixture goes through extraction and purification, sometimes using recrystallization from ethyl acetate or acetone, and then drying over vacuum to remove any solvent residues. Any impurity, especially unreacted starting material or remaining bromoalkane, gets traced by NMR and GC, keeping the product fit for sensitive applications.
Chemical Reactions & Modifications
The value of 1,3-Dimethylimidazolium Propane Bromide comes partly from what you can do with it as a platform. Chemists swap the bromide anion for other anions, like BF4−, PF6−, or NTf2−, generating new ionic liquids with tailored properties. Quaternization offers ways to tweak the imidazolium core, opening the door for derivatives with phosphonium, ammonium, or sulfonium for broadened solubility or binding. Its structure proves compatible with nucleophilic substitution, allowing modifications that adjust polarity, solubility, or even magnetic properties. Reactions using this salt as a catalyst report improved yields for stubborn alkylations or oxidations, especially for natural product derivatives. The compound finds use as a templating agent for zeolites, metal-organic frameworks, and sol-gel processes, exploiting its bulky, charged core.
Synonyms & Product Names
In catalogues and literature, you might spot this material under several names. Chemists often use 'C3ImMe2Br', shorthand for its core structure. Other variants include 1,3-dimethyl-1H-imidazol-3-ium propane bromide or simply DMIm-PrBr. Suppliers sometimes shorten it to 'imidazolium propyl bromide' for quick reference, but the CAS number remains the gold standard for identifying exactly what’s in the bottle.
Safety & Operational Standards
Handling safety ranks high, since the compound’s bromide content and potential for skin or respiratory irritation requires respect. Standard operating procedures call for nitrile gloves, safety goggles, and good ventilation, ideally a fume hood. Lab veterans never weigh it near open water sources, and always cap the bottle tightly thanks to its absorbent nature. Inhalation stays unlikely with solid samples, but spills or dust clouds call for immediate cleanup. Disposal guidelines lean toward incineration with halogen scrubbers, since bromide release can trigger environmental problems if mishandled. Shipping observes GHS labeling—irritant, non-flammable solid, and ‘handle with care’ tags—especially for international transfer.
Application Area
The real flexibility of 1,3-Dimethylimidazolium Propane Bromide stands out in application. Researchers in biomass processing use it as a cellulose solvent, breaking down stubborn plant matter that shrugs off regular acids or bases. Extraction chemists pull rare earth metals or pharmaceuticals from tough matrices using its phase-crossing behavior. Electrochemists load it into fuel cell electrolytes or redox flow batteries, noting high conductivity and low volatility. In catalyst design, the compound serves as a medium for organometallic catalysts, frequently boosting selectivity and yield thanks to its unique solvation shell. Green chemistry circles tout its use for cutting reaction times, reducing energy consumption, and lowering hazards compared to volatile alternatives.
Research & Development
Campus labs and major chemical companies devote significant R&D power to tweaking ionic liquids like this one. Studies now focus on chiral recognition, hoping to separate enantiomers for pharmaceutical use without costly chiral auxiliaries. Startups target CO2 capture or sequestration schemes, banking on the salt’s rich solubility and strong, tunable interactions. Analytical chemists improve separations, using the compound in HPLC or as a stationary phase for specialty applications. Work also revolves around refining scalability, cutting down on waste by continuous-flow synthesis or greener solvents. The challenge remains cost—making such ionic liquids in tons without sacrificing purity or performance.
Toxicity Research
Most published data show that 1,3-Dimethylimidazolium Propane Bromide skews toward low acute toxicity, especially compared to older solvents like dichloromethane or DMF. Toxicologists zero in on chronic effects and biodegradation, because the imidazolium core can persist in aqueous environments and may impact aquatic organisms if released untreated. The bromide counter-ion presents its own set of risks, especially in sensitive environments like wetlands or estuaries. So far, mammalian studies suggest limited bioaccumulation, but the lack of long-term, high-dose exposure studies means uncertainties stay on the table. Most guidelines still call for careful containment and controlled incineration, rather than disposal into normal chemical drains.
Future Prospects
Looking ahead, 1,3-Dimethylimidazolium Propane Bromide hangs onto its spot in the toolkit for sustainable chemistry. Demand from the battery and energy storage sectors could grow, especially as regulations phase out more hazardous solvents and push for renewable-powered manufacturing. Synthetic routes will likely tighten up, maybe with biocatalytic methods or more efficient recycling of bromide ions. Researchers already test blends with other ionic liquids, targeting new uses in microfluidics, polymer processing, and even nanomedicine. Success ultimately depends on how researchers manage scale, cost, and life-cycle safety, but the case for expanded use seems strong in an industry itching for cleaner, more versatile alternatives.
The Compound Behind the Name
1,3-Dimethylimidazolium Propane Bromide sounds like a mouthful straight out of a chemistry textbook. Beneath the long name sits a type of ionic liquid built from imidazolium. For years, lab researchers looked at these materials with fresh eyes because they bring something new to the table—unmatched stability, high ionic conductivity, and the kind of flexibility many industries need.
Powering Greener Chemistry
One of the things that excites many scientists about this compound is its role as a solvent. Ordinary solvents, like acetone or benzene, leave a heavy footprint. 1,3-Dimethylimidazolium Propane Bromide steps in as a cleaner, less volatile replacement. My experience with process design taught me that swapping out harsh chemicals makes a real difference. Fewer emissions, less risk for workers, cleaner waste streams. A research group in Germany ran tests on cellulose processing and found this ionic liquid worked wonders for dissolving the raw plant fibers into forms you could transform into bioplastics or films. The old petroleum-based solvents just couldn’t compete.
Better Performance in Energy Storage
In the scramble for better batteries, every detail matters: charge time, safety, and lifespan. Here’s where this compound shines. Manufacturers use it as an electrolyte or as an additive in lithium and sodium batteries. The ionic liquid's stability and low flammability help keep batteries running cooler and longer. Studies out of Asia show improved charge-discharge cycles in batteries featuring this substance. There’s a shift toward electric vehicles, portable electronics, and renewable energy storage. A safer, longer-lasting battery changes the landscape for everyone.
The Catalyst for Cleaner Reactions
My time in small R&D labs led me to appreciate just how valuable a versatile catalyst can be. That’s one of the hidden strengths of 1,3-Dimethylimidazolium Propane Bromide. In reactions like alkylation or esterification, this compound helps drive the process forward, delivering high yields with fewer byproducts. Researchers from India reported faster reaction times and purer outputs, which means less time and energy wasted. Cleaner reactions cut down on cleanup and toxic waste, bringing real benefits to both the environment and the folks working at the bench.
Challenges and Paths Forward
Not every solution is perfect. 1,3-Dimethylimidazolium Propane Bromide can carry a higher cost than older solvents. Large-scale producers often balk at switching, worried about price or supply chain hiccups. There’s also its fate after disposal to consider; scientists continue to probe the environmental impacts of ionic liquids. To push adoption further, manufacturers could invest in recycling systems for spent liquid or work with local authorities to create guidelines on their safe use and disposal.
Regulators play a part too. Promoting open data on health and ecological effects builds trust. Universities and research labs can help by developing cheaper synthesis routes or tweaking the molecule so it breaks down faster in the environment. The conversation doesn’t end at the factory gate. Safer, greener chemistry saves money only if the whole cycle, from production through disposal, gets some attention.
Concluding Thoughts on the Road Ahead
Each innovation in industrial chemistry shapes the future. 1,3-Dimethylimidazolium Propane Bromide stands as one of those behind-the-scenes tools that enables better outcomes in fields like bioplastics, energy storage, and green chemical processes. Progress in chemistry often means sweating the small details—less waste, better safety, smarter design choices. Listening to both lab data and real-world experience, people can make decisions that put health, safety, and sustainability first.
The Building Blocks
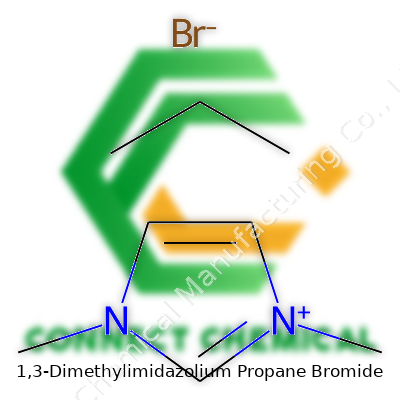
1,3-Dimethylimidazolium Propane Bromide shows up a lot these days, mostly in research circles focused on green chemistry solutions. This compound features a core called imidazole—picture a five-membered ring with two nitrogens and three carbons. In this version, two methyl groups lock onto the first and third nitrogen atoms, which brings about the “1,3-dimethyl” in its name. The ring then connects to a three-carbon chain—the “propane” piece—ending in a bromide ion.
Piecing all that together, the structure boils down to a cation with the 1,3-dimethylimidazolium core, a three-carbon tail, and bromide as the counter ion. The methylation at those two points influences both solubility and the overall charge distribution across the molecule.
Why This Structure Matters
People ask why a researcher or engineer might care so much about this arrangement. It’s because molecular tweaks like methylation and the specific chain length produce big changes in melting point, viscosity, and even how the substance acts with water or common solvents. That makes this compound part of the ionic liquids family—a new breed of fluids that don’t evaporate easily and can dissolve all sorts of things that water can’t handle.
I first learned about these chemicals in a lab focused on cleaning up polluted water. Our team explored how imidazolium salts could trap heavy metals. The 1,3-dimethylimidazolium group’s hydrophobic tail meant it hung onto certain toxins better than other salts. This real-world experience showed how a small change on a molecule made everything different at the scale of a whole experiment.
Concerns About Use and Future Directions
Using ionic liquids like this one comes with trade-offs. On paper, they offer low volatility—they stay put and don’t fill labs with toxic fumes like traditional solvents. Yet, there’s not enough known about how these materials interact with living things or move through soil and water. In some studies, imidazolium-based salts linger a long time in the environment. Their breakdown products sometimes cause as much trouble as petroleum distillates.
Transparency from manufacturers matters here. Responsible companies share safety data freely instead of hiding risks behind trade secrets. Academic labs need to follow suit. Sharing full chemical structures and open access data allows toxicologists and chemists to cross-check assumptions fast.
As for handling risk, smarter chemical design offers one avenue forward. Adjusting the length of the chain, swapping a methyl for something bulkier, or changing the counter ion can reduce toxicity and speed up natural breakdown when spilled. I’ve seen promising results from bio-based ionic liquids—these swap petroleum building blocks for renewable ones, offering similar properties without lingering environmental scars.
Chasing Better Solutions
It’s easy to see every new chemical as a quick fix, but choosing the right structure for the job calls for patience and open minds. Collaboration matters. Industrial users, regulators, and researchers need routine conversations about best practices and long-term impacts, not just quick wins in performance. For ionic liquids like 1,3-dimethylimidazolium propane bromide, the future looks brightest when we balance lab innovation with real-world caution and clear communication.
A molecular tweak can open doors to new technologies or bite back in ways nobody expected. The best results often come from mixing careful study, honest data sharing, and staying grounded in both chemistry and the bigger picture of how our inventions ripple through the world outside the lab.
The Chemical in Question
1,3-Dimethylimidazolium propane bromide gets attention in labs and industrial settings that work with ionic liquids. It belongs to a class of chemicals valued for their use in chemical processes, extraction technologies, and sometimes as solvents in research. Because new chemicals keep appearing in modern labs, safety questions always follow.
Toxicity and Human Health
People eye ionic liquids for their low evaporation and perceived lower flammability, often compared to older solvents. Still, the assumption that “less flammable” equals “less toxic” can mislead. Scientific studies show some imidazolium compounds carry real toxicity risks for both humans and aquatic organisms. For example, research published in journals including Chemosphere and Journal of Hazardous Materials reports moderate toxicity in aquatic systems from imidazolium-based ionic liquids. Toxicity grows in significance with the size of the alkyl chain, but even short-chain versions like 1,3-dimethylimidazolium propane bromide show effects not just to small aquatic critters, but in cellular tests as well.
I remember handling similar ionic liquids in research environments. Gloves, goggles, and fume hoods always stayed close by because these chemicals did not have the long-term safety data you get with more common solvents. Risk stands higher for newer or less studied compounds. Absorption through skin or accidental inhalation can lead to unknown outcomes, and with little data on chronic effects, that uncertainty feels risky.
Environmental Concerns
After lab use, disposal poses questions. Ionic liquids often stick around in the environment. The imidazolium ring structure does not degrade as quickly as, say, table salt or acetone. Some studies show that bromide ions can harm soil and water organisms. Researchers from Germany and China have tracked persistent residues after spills and improper waste treatment. You release too much into local waterways, and toxicity adds up—fish, crustaceans, and algae start paying the price.
Worker Safety
Safe lab practice means not taking shortcuts. Without reliable data sheets and clear hazard labels, mistakes can happen easily. For those working in chemical plants or research spaces, skin irritation and eye damage stand as plausible risks when using 1,3-dimethylimidazolium propane bromide. Ingestion, accidental or otherwise, invites trouble because no antidotes or long-term antidotes exist for this class of compound.
Safer Handling and Regulation
Some might think calling chemicals “green” due to low volatility means the problems are gone. Direct evidence points out these assumptions miss the mark. In practice, smart scientists and industrial safety officers rely on regular training, personal protective gear, and secure chemical storage. Waste disposal should pass through approved hazardous waste programs, keeping waterways and landfills safe.
Government agencies, including US EPA and the European Chemicals Agency, suggest treating new ionic liquid chemicals with caution. Registrants must back up new chemicals with toxicity studies and disposal recommendations that go deeper than general statements. Pressure is growing for chemical companies to test for long-term environmental persistence and toxicology before rolling out these compounds at scale.
Moving Forward
Nobody wants to stifle progress in green chemistry, but ignoring toxicity data invites harm to people and ecosystems. I keep a healthy skepticism anytime a new product arrives without robust safety information. Until more is known, lean on the side of caution—protective equipment, strict cleanup procedures, and a habit of respecting unknowns.
Understanding the Risks and Responsibilities
Handling any chemical—especially ionic liquids like 1,3-Dimethylimidazolium Propane Bromide—demands respect for both safety and real-world consequences. Some folks see these imidazolium compounds as modern miracle workers. They pop up in labs as solvents, catalysts, and even participants in energy storage research. Yet, no matter how impressive a material performs, storage forms the backbone of safe use.
My time working in university research taught me early that shortcuts with chemicals bite back. Bottles labeled with a dash of tape instead of real warnings led to headaches and twice as much wasted product after someone ignored desk instructions. One slip in safety habits means ruined experiments or, worse, a trip to the ER.
Don’t Play Fast and Loose—Humidity and Heat Will Ruin Your Day
1,3-Dimethylimidazolium Propane Bromide soaks up moisture from the air like a sponge. Leave a bottle open or stored in a humid closet, and it eventually clumps up, starts to degrade, or even forms hazardous byproducts. I’ve seen careless storage turn perfectly good chemicals into an ugly, unusable mess. Tossing money down the drain isn’t just inconvenient, it sometimes causes problems with sensitive reactions or unexpected toxicity.
Heat presents another threat. This salt stays stable at room temperature but can break down if left near radiators or heat sources. Once decomposition starts, you face new dangers—think strong smells, altered reactivity, or the risk of skin and lung irritation. OSHA reports highlight cases where improper storage lead to chemical burns and respiratory distress. Keeping the material out of direct sun and away from heaters makes a big difference in avoiding those kinds of mishaps.
Poor Storage Spreads Problems Across Teams
Bad chemical storage isn’t just a personal issue. Someone’s forgotten vial tucked behind a stack of cardboard boxes sparks confusion during inventory checks, sometimes leading to panic when a label fades or a cap breaks off. Broken containers and spills translate to wasted time, messes that require special cleanup, and loss of trust within a team. The worst offenders create a legacy of disorganization that slows down everyone who steps into the lab.
Practical Steps Everyone Can Follow
Solid habits make the difference. Keeping 1,3-Dimethylimidazolium Propane Bromide in tightly sealed glass or high-quality plastic containers preserves its integrity. Dry environments, like cabinets with desiccant packs, add a layer of protection against moisture. Anyone handling larger amounts should think about lockable, labeled cabinets far from sunlight or fluctuating temperatures.
Label every single container, even if you’re the only one using it. Double check for chemical incompatibilities: store away from acids, oxidizers, or anything that could trigger a reaction. Institutional policies often suggest secondary containment—simple trays or bins—to catch leaks before they spread. Training isn’t glamorous but pays off. A team that understands the risks looks out for one another, spotting cracked lids or condensation before those little things become disasters.
Building Skills and Awareness Pays Off
Learning by habit, not by accident, keeps labs productive and coworkers safe. Anyone new to handling ionic liquids benefits from real guidance and hands-on practice, while seasoned researchers serve as unofficial coaches. The effort spent building safety routines pays off, not just in clean shelves and clear labels but in keeping injuries and product losses far from the workbench. Chemicals like 1,3-Dimethylimidazolium Propane Bromide offer promise, but only if users carry responsibility through every step—from delivery to storage, and beyond.
What Stands Behind This Unusual Compound?
Interest in specialty solvents and ionic liquids started spiking around the early 2000s, and 1,3-dimethylimidazolium propane bromide stepped out from a crowd of similar molecules. This compound belongs to the family of imidazolium-based ionic liquids—the kind of materials that let you drop the boiling point, play with polarity, and cut down on volatility. For many labs and startups, these features sound less like jargon and more like workable benefits.
Solving Real Problems in Organic Chemistry
It’s easy to picture an organic chemist tuning the details of a reaction and getting fed up with old-fashioned, flammable solvents. 1,3-dimethylimidazolium propane bromide is set up to play a part in green chemistry. With this ionic liquid, you get a substance that barely evaporates at room temperature and often helps reactions run cleaner. Not many solvents tackle stubborn tasks like dissolving cellulose, but this compound fits the bill.
Some of the biggest research schools now use ionic liquids to extract plant compounds, separate chemicals, or carry out tricky syntheses. Anyone who’s spent hours tweaking process conditions to boost yields will understand why swapping out a hazardous solvent for a safer alternative matters to health and the bottom line. One study showed that this compound pulled lignin and hemicellulose from agricultural waste without giving off the usual hazardous fumes, making the job easier for both chemists and the planet.
Helping Electrochemistry and Material Science
Material scientists picked up 1,3-dimethylimidazolium propane bromide thanks to its stability and ionic conductivity. Folks working in energy storage devices, such as supercapacitors or batteries, look for safe ways to carry ions between electrodes. Standard solvents get flammable or break down easily, but this substance holds out under stress and heat. A growing group of research articles supports its use in developing safer, longer-lasting energy devices.
There’s also talk about using this ionic liquid for electrodeposition. Instead of using water-based or traditional organic media, labs can try ionic liquids to plate out metals at lower temperatures or get purer coatings. In plating microelectronics, for example, these features help reach finer detail while skipping harsh chemicals.
Cleaning Up Laboratories and Factories
One pressing need across labs and manufacturing is to reduce hazardous waste while maintaining performance. The design of 1,3-dimethylimidazolium propane bromide helps cut down the need for volatile organic compounds. After main reactions or processes, the compound often sticks around, making recycling more efficient. One textile processing group tested this compound for dye uptake; the results showed higher efficiency while trimming down both water and solvent use.
Pushing for Responsible Use
Regulatory bodies want to see less impact from chemical waste. For this compound, long-term health effects still need closer tracking, but its promise in lowering toxicity has pushed some manufacturers to give it a try. Academic teams have called for detailed life-cycle analyses and real-user feedback before mass adoption.
For anyone working in R&D, the big shift involves seeing 1,3-dimethylimidazolium propane bromide as a workhorse rather than just a specialty solvent. Its impact touches everything from agency regulations to safety data sheets on lab benches worldwide—and for people who value results that speak for themselves, there are plenty of reasons to keep experimenting.