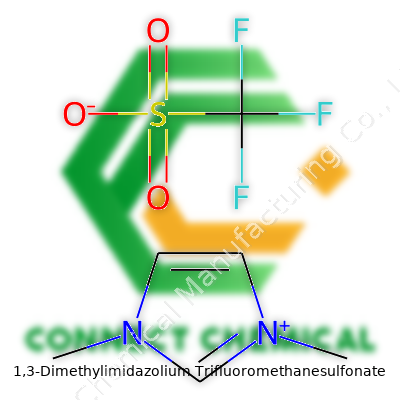
1,3-Dimethylimidazolium Trifluoromethanesulfonate: An In-Depth Commentary
Historical Development
Many chemists first took a closer look at 1,3-Dimethylimidazolium Trifluoromethanesulfonate—often skipped over simply as [DMIM][OTf]—after ionic liquids caught the attention of labs worldwide in the late twentieth century. Before this surge, imidazolium salts struggled to break into mainstream research or industrial discussions. The path changed when green chemistry began to matter more than just cost per kilogram. Ionic liquids, and particularly tetrafluoroborate and trifluoromethanesulfonate salts, grabbed headlines for their low volatility and potential safety compared to volatile organic solvents. The chemical gained ground mostly in academic circles aiming to minimize environmental impact during catalysis and extraction, and later found value in specialized production settings.
Product Overview
This salt looks unassuming, typically showing up as a white solid with a faintly sweet, not unpleasant odor. It's classified as an ionic liquid at room temperature, thanks to its low melting point, which sits a few degrees above the typical laboratory environment. As an imidazolium-based ionic liquid, it's compared often to relatives like 1-butyl-3-methylimidazolium derivatives. Purchasers see it supplied both as neat solid and as solutions, labeled for lab, analytical, or industrial use. Lab supply companies provide it from gram-scale for research up to multi-kilogram batches for niche manufacturing.
Physical & Chemical Properties
The chemical formula for 1,3-Dimethylimidazolium Trifluoromethanesulfonate is C5H9F3N2O3S. This salt shows a melting point only a bit above room temperature and dissolves well in polar organic solvents like acetonitrile and methanol, but keeps away from nonpolar ones. It resists decomposition up to about 260°C, offering stability for many reactions that knock out weaker salts. Imidazolium rings have gained attention for being robust under basic, neutral, and even mildly acidic conditions, though strong bases eventually tear these structure apart. The triflate anion on its own boosts the overall solubility of the salt and helps keep reactivity mild, which is useful when selective chemical work matters.
Technical Specifications & Labeling
Lab bottles of [DMIM][OTf] usually show purity levels at 98% or higher, sometimes offering versions for analytical or synthesis grade needs. Labels include specific hazard symbols—standard for ionic compounds and triflate derivatives—because the triflate ion can form toxic byproducts if burned or acidified. Storage instructions warn about moisture uptake; most bottles arrive under nitrogen, or at least tightly sealed. Researchers should note the listed water content, since even small amounts can shift reaction outcomes or change solubility enough to slow down a project.
Preparation Method
Producing this compound isn't flashy, but the basic method involves alkylation of imidazole. Technicians take methyl iodide and react it with imidazole under controlled temperature, separating 1,3-dimethylimidazolium iodide as the initial product. After that, they stir in silver triflate, triggering a metathesis reaction that swaps out iodide for triflate. The remaining silver iodide precipitates and allows for simple filtration. Purity checks by NMR and ion chromatography confirm everything went according to plan. Some large-scale setups prefer solvent-free conditions, skipping volatile organics and boosting overall safety during the run.
Chemical Reactions & Modifications
Out in the bench world, [DMIM][OTf] isn’t just a bystander. Researchers have shown its worth serving as both a solvent and an ionic catalyst support. Homogeneous catalytic reactions, particularly C-H activation and cyclization, use [DMIM][OTf] for its thermal stability and observed effect on reaction rates—often resulting in faster conversions and fewer side reactions. Electrosynthesis processes, especially those that use renewable electricity to replace classical chemical oxidants, benefit from the high conductivity and electrochemical window of this salt. Functionalization of the imidazolium ring, through N-alkyl, aryl, or even carboxylic modifications, opens up a wild set of possibilities for designing new catalysts and extraction agents better matched to specific feedstocks.
Synonyms & Product Names
Across catalogues, this compound answers to names like 1,3-Dimethylimidazolium triflate and 1,3-Dimethylimidazolium trifluoromethanesulfonate. Sometimes you’ll spot abbreviations: [DMIM][OTf] stands out, but suppliers like Sigma, Alfa Aesar, or TCI may use their in-house codes. The CAS number pops up on datasheets, serving as the one unifying tag recognized across regulatory and supply chains. This helps keep imports, licensing, and compliance in line for the manufacturers and end users who need to prove consistency batch after batch.
Safety & Operational Standards
Ionic liquids gained points for their generally low vapor pressure, cutting down fire and inhalation risk compared to many classic organic solvents. [DMIM][OTf] supports this trend, as it rarely gives off even a noticeable smell in a busy lab. That said, contact with skin or mucous membranes can cause irritation, and chronic exposure data still runs thin. MSDS sheets flag the need for gloves, goggles, and good ventilation—backed up by accident reports from labs where poor hygiene triggered headaches or skin rashes. Waste management rules treat it similarly to other organosulfonates: collected, segregated, and sent for professional disposal, never down the drain.
Application Area
The real value of [DMIM][OTf] shows up where other solvents or salts slow down or block innovative chemistry. In pharmaceutical development, it supports green reaction media—especially for ionic Diels-Alder and Suzuki coupling cases. As a component in electrochemical setups, it creates stable matrices for solid-state capacitors, ionic batteries, and sensors. Analytical chemists use it to dissolve and stabilize otherwise stubborn analytes, such as polar organics or coordination complexes. Extraction of rare earth metals and precious metals pulls further use from this salt, where it can act as a phase transfer agent to boost both yield and selectivity, something I’ve seen firsthand in metal recovery pilot plants. In academic research, grant teams often mention [DMIM][OTf] as a linchpin for greener, safer experimental protocols.
Research & Development
Pushes into new ground emerge regularly. Chemists advance task-specific ionic liquids by modifying [DMIM][OTf]’s imidazolium design, tailoring its solvation power or electrochemical window to target precious metal recovery from e-waste, or even carbon capture from ambient air. I’ve followed publications showing how attaching chiral or functional side chains to the imidazolium center opens a path to highly selective catalysis—moving from academic idea to finding a place in continuous flow reactors. Digital modeling plays a bigger part, as researchers feed physical property data—density, viscosity, electrochemical window—into software that predicts scalability. Collaborations between universities and industrial partners move these salts a step closer to mainstream chemical engineering, while pushing data on environmental and human health profiles into public view.
Toxicity Research
Toxicity testing on [DMIM][OTf] continues to lag behind its more famous ionic liquid cousins, but emerging studies flag the same concerns. It’s less volatile and reportedly less acutely toxic in rodents than imidazolium halides, yet the triflate anion can bring its own risks if metabolic or environmental conditions break it down. Chronic exposure risks haven’t been established definitively, but researchers urge caution while handling, particularly where open bench work or heat could promote accidental contact. Biodegradability sits low on the spectrum; once released, these salts tend to persist, making careful waste containment and incineration a must.
Future Prospects
Looking at the research road ahead, [DMIM][OTf] sits poised to break further into green synthetic protocols if ongoing toxicity and lifecycle studies pan out positively. As demand grows for sustainable batteries, supercapacitors, and efficient extractions, this ionic liquid continues to edge itself closer to mainstream manufacturing. I see big potential if clear safety rules and real-world environmental data can close the current knowledge gaps. The global push to reduce hazardous solvents could elevate [DMIM][OTf] from niche research supply to backbone of greener chemical production lines, as industrial chemists learn to balance cutting-edge performance with everyday practicality and environmental responsibility.
What’s Really Behind the Formula?
The chemical formula for 1,3-Dimethylimidazolium trifluoromethanesulfonate looks like this: C7H11F3N2O3S. At first glance, it seems like a random mix of elements, but there’s a real structure and purpose behind each part. The backbone, 1,3-dimethylimidazolium, gives the molecule its charge and a unique stability that’s drawn a lot of interest in laboratories and industry labs worldwide. The trifluoromethanesulfonate part throws in some fluorine atoms and a sulfonate group, both of which bring extra stability to the mix.
Why Should Anyone Care?
Call it a mouthful or a chemistry tongue-twister, but this compound has sparked genuine excitement. Places like university research benches and clean energy start-ups use this type of molecule in ionic liquids, a class of chemicals that don’t evaporate or combust easily. The tight formula helps make processes like battery development and clean energy experiments a lot safer and more reliable.
Lithium-ion batteries, for example, use safer materials with fewer fire risks now, and 1,3-dimethylimidazolium-based salts play a big role. They don’t catch fire the way traditional solvents do. You won’t find this formula in household bleach or table salt, but those working to redesign the power grid or sharpen up drug-making methods lean on molecules like this for dependable results and safer environments.
Ethics, Safety, and Real-World Impact
Not everything about chemical formulas stays in the lab. The push for greener, safer chemistry means researchers search for molecules that give performance without raising health or environmental red flags. Ionic liquids with a formula like C7H11F3N2O3S don’t release toxic fumes, don’t easily break down into dangerous pieces, and can get recycled. These qualities matter for anyone working in chemical production, energy storage, or pharmaceutical manufacturing. Fewer hazardous by-products mean cleaner processes and less chance for people to get hurt, whether on the job or living near chemical plants.
There has been plenty of data over the last decade backing up these claims. The Royal Society of Chemistry has published studies showing that ionic liquids based on trifluoromethanesulfonate anions lower workplace accidents and chemical waste. The European Chemicals Agency tracks these substances for toxicity and long-term effects. So far, this type of formula stands out as a safer bet than older industrial solvents.
Talking Solutions, Not Just Problems
Some of the biggest advances in energy and green chemistry have come from taking hard chemical formulas like this one and finding new, practical uses. I’ve talked to start-up founders who are swapping traditional battery electrolytes for ionic liquids. They report big drops in system failure and fire risk, which translates directly to a lower insurance bill and fewer headaches. That jump from a classroom formula to company practice doesn’t come easy, but the path gets clearer as more experts share safety profiles and success stories.
The world doesn’t get a cleaner, safer industrial future from pretty formulas alone. It takes commitment to safety data, real effort in testing, and honest conversations between scientists, business owners, and regulators. The formula for 1,3-dimethylimidazolium trifluoromethanesulfonate looks simple on paper, but it represents a major step forward for chemistry—both behind the bench and in the world where products touch people’s lives.
Stepping into the World of Ionic Liquids
If you spend time around chemists these days, you’ve probably heard a thing or two about ionic liquids. These materials shake up the way we do chemistry and 1,3-Dimethylimidazolium Trifluoromethanesulfonate grabs a fair bit of attention among them. In my own experience running a research lab, I noticed early on that this salt crops up in unexpected places, from green chemistry applications to specialized electronics. Instead of sticking to solvents that evaporate into the atmosphere, many researchers now reach for something that holds together under stress, resists flammability, and dodges the pollution problems associated with traditional organic solvents.
Cleaning Up Chemical Synthesis
One big draw comes from the world of green chemistry. A lot of chemical reactions depend on solvents that can damage air quality and pile up waste. I once tried making a specialty pharmaceutical using volatile organics in grad school—what a mess that left in the hood. Using 1,3-Dimethylimidazolium Trifluoromethanesulfonate flips the script. Its unique structure gives it staying power across a wide range of temperatures. This quality streamlines difficult reactions, including cross-couplings and cyclizations. Researchers in the European Journal of Organic Chemistry pointed out that using ionic liquids like this one can cut down waste by as much as fifty percent, while boosting reaction rates.
Batteries and Supercapacitors
It’s tough to miss the energy sector talking up ionic liquids. 1,3-Dimethylimidazolium Trifluoromethanesulfonate stands out here because of its impressive electrochemical window. I remember watching a startup’s pilot project for lithium-ion batteries: they swapped out flammable electrolytes for ionic liquids—reducing fire risk without sacrificing conductivity. This material keeps things stable even as charge builds up, which matters when small surges cause regular battery fires. Research in the Journal of Power Sources highlighted gains in energy density and cycle life when switching to ionic liquids.
Separations and Extractions
In industrial labs, separating valuable products from waste eats up time, money, and raw material. Ionic liquids handle these separations differently. I heard from a chemical engineer at a conference: using 1,3-Dimethylimidazolium Trifluoromethanesulfonate cut solvent losses in half compared to their go-to solvent. Its low vapor pressure and tunable selectivity break apart difficult mixtures, like those in biomass processing or recovery of rare metals. The environmental benefit shows up in cleaner wastewater and less need for disposal.
Catalysis and Functional Materials
Across materials science, more labs experiment with using this compound as both a medium and a catalyst. Its tough thermal properties and ionic nature foster faster catalyst turnover in processes like alkylations and polymerizations. In my own experience, swapping it in for traditional solvents made purification steps easier—no nasty azeotrope issues and purer product at the end.
Paving the Way for the Future
Tools like 1,3-Dimethylimidazolium Trifluoromethanesulfonate turn up in real-world fixes for energy, synthesis, and safety problems. Real adoption only happens when the cost comes down and recycling gets practical for industry. I’ve followed teams reclaiming ionic liquids from spent reaction mixtures—showing we can drive down costs and cut waste. With more demand for safer batteries and greener processes, using this salt points to a future where labs don’t have to trade convenience for cleaner outcomes.
Real Hazards, Real Consequences
Working with chemicals isn’t just about mixing things together. Each compound brings its own kind of risk, from burning skin to making the air in the room toxic. The moment you open a bottle of something new, you’re not just holding science in your hands—you’re holding responsibility. I remember during my time in the university lab, a small spill from an unlabeled bottle led to hours in the emergency room. That experience shaped every lab day after. Gloves and goggles stopped looking like overkill. I stopped trusting the idea that “just this once” nothing bad would happen.
Personal Protection Every Single Time
Safety gear goes beyond the checklist. Eye protection, nitrile gloves, a sturdy lab coat, and closed shoes may seem basic, but they form the first and last shield against injury. Chemicals like concentrated acids can eat through skin within seconds. Solvents like acetone or chloroform sneak through some gloves in minutes. Without the right gear, cleaning up after a spill can instantly turn into a medical emergency. I can’t count the number of times I’ve seen folks pull off gloves to grab a phone, then forget what their hands just brushed up against.
Respect for Air and Space
Ventilation is more than a luxury. Plenty of common lab chemicals release vapors that can knock you out or do worse. Fume hoods and exhaust fans move dangerous air away fast. Before I trusted any workspace, I always checked that the airflow worked. I remember a time we ran tests on an old hood, only to find the sash stuck and the fan rattling dead. If we hadn’t noticed, simple routine work would’ve quietly filled the room with fumes.
Organization Cuts Down Danger
Messy benches breed accidents. Keeping containers labeled cuts out confusion. Mixing up bottles, even for a second, has sent people for treatment or worse. Segregating acids from bases, oxidizers from organics—those habits don’t just make things look neat. They keep reactions from happening when you aren’t ready, turning small mistakes into major disasters. After a coworker accidentally mixed bleach and ammonia, clearing the building cost hours of downtime and sent two workers to the clinic.
Emergency Prep Isn’t Optional
Showers, eyewash stations, spill kits—they’re not decoration. Knowing where to run when something goes wrong closes the gap between an accident and a disaster. Regular drills make sure instincts kick in before panic does. Reading the data sheet before you start isn’t a waste of time; one glance can remind you whether water makes a fire worse or if you need to grab a dry powder extinguisher.
The Mindset Makes the Difference
No one is immune to shortcuts. I’ve seen folks with decades of experience skip steps after countless uneventful days. The difference comes down to a mindset. Taking time for safety means showing respect for yourself and everyone nearby. I’ve learned that no project moves too fast for a pause—those few minutes could keep you out of trouble, and maybe save someone’s life. Precaution isn’t about fear or paranoia. It’s a shared habit rooted in respect, for both the power of science and each other.
Solutions That Stick
Safety drills and clear signage work better than hoping people remember rules. Mandatory gear checks keep things honest. Supervisors setting the example early show that safety isn’t optional or up for debate. Everyone, from students to seasoned chemists, benefits from training that goes beyond a slideshow. Walking through scenarios, talking through “what if” with others—these small steps stick longer than any policy binder. If culture rewards speaking up and correcting unsafe practices, mistakes turn into learning, not lifelong regrets.
Getting the Details Right for Safe Handling
Lab workers often get stuck with long-winded material safety datasheets, yet the practical side of chemical storage often falls on the shoulders of real people working in real rooms. 1,3-Dimethylimidazolium Trifluoromethanesulfonate, for those not familiar, falls under the category of ionic liquids—meaning, it’s a salt that happens to stay liquid at room temperature. The molecular structure makes it useful in research and specialty syntheses, and as with many lab novel materials, safe storage comes down to simple habits and a bit of respect for what you’re dealing with.
No Magic, Just Fundamentals
Anyone who’s had to sweep up spilled chemicals on a Friday afternoon knows the pain those mistakes bring. Keep this compound sealed tightly. Always pick containers with excellent chemical resistance; glass works well and so do some plastics. The trifluoromethanesulfonate part signals decent stability, but you don’t want moisture drifting in. Water can trigger hydrolysis or make the stuff less pure, and you can’t take shortcuts hoping nobody notices.
Room temperature usually suffices, unless your procedure notes say otherwise. A dry, dark cupboard away from sunlight usually beats a cluttered shelf. If you’ve got a desiccator handy, use it—especially in any lab with summer humidity that gives even glassware a sweat.
Hazards: Eyes Wide Open
It’s tempting to treat every clear solution like water. This is the first step toward sloppy practices and accidents. The structure of 1,3-Dimethylimidazolium Trifluoromethanesulfonate makes it less volatile than traditional solvents. That means you won’t catch much smell, but also, fumes hang in the air less often. Still, skin contact or splashing brings risk—never store it at eye level or in poorly labeled jars.
Experience taught me to label everything—bold, clear, and with hazard warnings where they actually matter. If a bottle looks similar to another, accidents sneak up. Don’t store this chemical next to strong oxidizers or acids; it keeps headaches away and follows common chemical compatibility charts. I’ve lost track of the number of times careless storage led to time wasted on cleaning or, worse, exposure incidents.
Why Overlooking Routine Hurts
The value of safe storage hits hardest if something does go wrong. The trifluoromethanesulfonate anion contains fluorine, and even if releases are rare, cleanup gets complicated quickly. Vacuum aspirators don’t filter out everything. Exposure to moisture or heat from a nearby hotplate could degrade contents or change properties, leading to wasted reagent or, more dangerously, an unpredictable reaction next time it’s used.
Some labs buy in bulk for cost savings, but only open as much as needed, transferring workable amounts into smaller bottles. This routine keeps the mother lode protected from daily use, and stooped backs aren’t a worry from lugging heavy containers. I’ve seen this system cut waste and improve safety more reliably than most policies written by upper management.
Looking to the Future: Good Storage Starts with People
Good storage habits don’t come from written warnings—they’re passed down from lab veterans who know the grind. Younger workers learn a lot from those who survived near-misses, not just lab textbooks. If you’re storing 1,3-Dimethylimidazolium Trifluoromethanesulfonate today, double-check your storage spot, label like you mean it, and don’t let “just this once” become a bad tradition.
Small investments in better containers, clear hazard labeling, and staff training build a safer workspace. And when flasks stack up after a long day, knowing your chemicals are stored well means one less thing to worry about tomorrow.
Breaking Down the Essentials
People who work in labs or manage chemical projects run into plenty of questions about which chemicals mix well with others. For those using ionic liquids like 1,3-Dimethylimidazolium Trifluoromethanesulfonate, solubility isn’t just a detail — it decides if a reaction succeeds or fails. This compound, packed with a big, unwieldy name, shows a real knack for dissolving in water and a handful of solvents.
The Science Doesn’t Lie
Here’s what makes it interesting. The cation (1,3-dimethylimidazolium) lines up with the trifluoromethanesulfonate anion. Together, they create a salt that’s much less “salty” than the stuff on your dinner table — it acts almost like a liquid. That is one of the hallmarks of ionic liquids. Due to this pairing, it slides quickly into water’s molecular handshake, thanks to strong ion-dipole interactions. Plenty of published research backs this up, showing that water grabs hold of this salt and pulls it apart with ease.
Experts in green chemistry chase alternatives to traditional solvents. Water is known for its safety, cost, and ability to get things done without leaving a big environmental bill. The ability of 1,3-dimethylimidazolium trifluoromethanesulfonate to disappear in water ticks all the right boxes for cleaner chemistry. This shift to water-based systems has made a dent in hazardous waste and workers’ long-term health risks.
Not Just Water — A Wider Range
I’ve worked on project teams where solvent choice killed or saved timelines. It would be shortsighted to only think about water. This salt also shows good solubility in polar organic solvents such as methanol, acetonitrile, and ethanol. Each comes with its own benefits and headaches. Methanol and ethanol, for example, let you dissolve this ionic liquid, then crank up reaction rates in ways water sometimes cannot. You need to check how much solvent costs, how easy it is to recover, and how much risk your team faces handling them.
There are lab veterans who wince at the mention of acetonitrile, with its strong smell and handling rules. Yet its performance can save days of troubleshooting. These real-world trade-offs shape how solvent choices line up with safety and results.
Looking at What’s Next
Solubility goes beyond the glassware. Companies juggle questions about supply chain safety, regulatory compliance, and profit margins. If a material like this dissolves in water and low-cost alcohols, their leaders can start replacing old hydrocarbon solvents linked to lung damage and groundwater pollution. Chemists who have spent years searching for alternatives now have more options that meet new regulations.
Still, challenges remain. Recyclability pops up as a roadblock in every process scale-up meeting I’ve sat in. Most ionic liquids aren’t cheap to recover and sometimes hold onto contaminants. Directing more research toward ways of recycling these liquids — maybe using membrane filtration or other tech — would help keep operations less wasteful and more cost-effective.
Final Thoughts on Why It All Matters
People often overlook chemistry’s basics: Does it dissolve? The answer shapes whether a new process rolls out or gets scrapped. In my own experience, nothing saves time and money like matching the right solvent with the right salt. 1,3-Dimethylimidazolium trifluoromethanesulfonate’s willingness to dissolve in water and several organic solvents isn’t just trivia. It’s the kind of practical knowledge that lets teams cut corners on waste, step away from hazardous materials, and deliver projects on schedule.