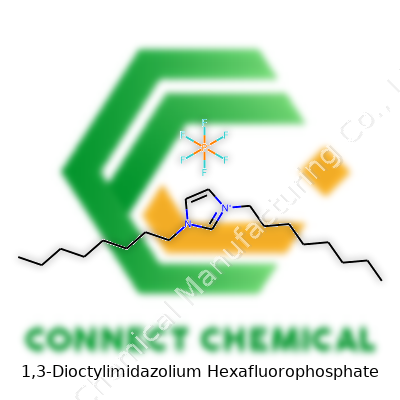
1,3-Dioctylimidazolium Hexafluorophosphate: A Deep Dive
Historical Development
People didn't always think of ionic liquids as more than just chemical curiosities. The story behind 1,3-Dioctylimidazolium Hexafluorophosphate traces back to the evolution of ionic liquids as alternatives to volatile organic solvents. In the late twentieth century, labs started shifting focus as regulatory and safety concerns began pushing industry away from hazardous solvents. Chemists searching for new compounds stumbled across imidazolium-based ionic liquids, and quickly, several teams realized their potential. Over time, the structure of 1,3-Dioctylimidazolium—with two long octyl chains attached—opened doors to new solubility profiles and a better match for more hydrophobic reaction environments. Researchers with an eye for green chemistry started tuning these ionic liquids, adjusting their properties with different substituents and swapping anions such as hexafluorophosphate in pursuit of stability and performance. The technological shifts in synthesis, along with advances in high-throughput screening, drove increasing adoption in academic and industrial circles.
Product Overview
Walking through a chemical supplier’s catalog, 1,3-Dioctylimidazolium Hexafluorophosphate stands out thanks to its long aliphatic tails and the PF6- anion. Folks working on catalysis, extraction, or battery technology pay close attention to the cation’s hydrophobic nature and the compound’s stability compared to classic imidazolium salts. People notice a low melting point, often liquid at room temperature, and robust electrochemical stability. As demand grows for solvents that avoid flammability and high vapor pressure, this ionic liquid jumps onto the shortlist for process engineers and lab chemists alike. Different supplier specs mention varying degrees of water content or purity, so buyers often test new batches for trace halides or metallic impurities before moving to scale-up runs.
Physical & Chemical Properties
Ask a synthetic chemist, and they’ll tell you the value lies in the unique attributes. 1,3-Dioctylimidazolium Hexafluorophosphate doesn’t evaporate easily, thanks to negligible vapor pressure. The compound’s viscosity trends higher with longer alkyl chains, so stirring and mixing takes real attention in the lab. Density sits above most organic solvents, and while transparent, the liquid can pick up a faint yellow hue if left open to air. Hydrophobic characteristics stem from those long alkyl chains, and PF6− brings strong resistance to hydrolysis in neutral and mildly acidic conditions. Electrical conductivity falls between classic salts and non-polar solvents, which means electrochemical engineers can tune cell architectures in energy storage devices. In practical work, the liquid’s stability even at elevated temperatures invites new uses, though some decomposition forms HF if heated with water present—an operational hazard that labs handle with respect.
Technical Specifications & Labeling
Labels matter as much as the product itself. Any bottle shipped from a reputable supplier highlights both chemical purity and residual water by Karl Fischer titration. You see CAS numbers, batch identifiers, production dates, and safety pictograms all over labels and MSDS sheets. Because trace chloride or halide residues from synthesis impact some catalysis reactions, top suppliers provide impurity spec sheets and offer re-drying services for moisture-sensitive work. Packaging consists of airtight amber glass or PTFE bottles, often wrapped with secondary containment to manage leaks during transport. As a customer, it's wise to cross-verify molecular weight, melting point, and key spectroscopic signatures (NMR, IR) listed on datasheets against fresh incoming product before committing to major experiments.
Preparation Method
People in research settings often prepare 1,3-Dioctylimidazolium Hexafluorophosphate through multi-step synthesis. Work usually starts with 1-methylimidazole, which reacts with 1-bromooctane under reflux to attach the octyl chains, giving 1,3-Dioctylimidazolium bromide. The process usually proceeds under nitrogen to limit side oxidation. Chemists then perform a metathesis reaction, stirring the intermediate bromide with potassium hexafluorophosphate in water or acetonitrile, leading to precipitation of KBr and isolation of the ionic liquid. Purification hinges on thorough washing and repeated extractions, sometimes using silica filtration or even vacuum distillation for solvent removal. Yield and purity depend heavily on reaction conditions, the grade of starting materials, and vigilance during washing steps to remove residual halides that sabotage sensitive downstream applications. Direct handling of PF6− requires training, since poor technique can introduce HF contamination, especially if the process occurs in a humid room.
Chemical Reactions & Modifications
Imidazolium-based ionic liquids like this one show broad tolerance for a range of modifications. Chemists use the nitrogen in the imidazole ring as a handle for post-synthetic tweaking, adding more hydrophobic tails or modifying the aromatic ring to change solubility. The liquid can stabilize metal catalysts that would decompose in water or regular organic solvents. Researchers experiment with swapping out the PF6− anion for alternatives like BF4− or NTf2− to alter hydrophobicity or conductivity, all while maintaining the core imidazolium structure. In reactive extractions, the ionic liquid acts both as a solvent and a phase transfer catalyst, dissolving reagents that wouldn’t normally mix. More recently, labs focus on immobilizing this ionic liquid on solid supports to create hybrid materials for catalysis and separations, exploring surface chemistry that links the imidazolium headgroup to silica or carbon scaffolds.
Synonyms & Product Names
Chemical suppliers and research papers use several names: 1,3-Dioctylimidazolium hexafluorophosphate, C8C8imPF6, or a host of abbreviations like [C8C8im][PF6]. Some catalogs reference “Bis(octyl)imidazolium hexafluorophosphate,” though experienced chemists know to double-check the structure. In less formal settings, it goes by “dialkyl imidazolium ionic liquid” in reference to the hydrophobic cation core. Each supplier uses slightly different trade or inventory numbers, which can cause headaches unless a researcher cross-references CAS numbers and supplier codes before placing bulk orders.
Safety & Operational Standards
Lab veterans treat this compound with the care it demands. Despite low volatility and reduced fire risk compared to classic solvents, 1,3-Dioctylimidazolium Hexafluorophosphate doesn’t mean people can ignore hazards. Gloves, splash goggles, and a fume hood come standard. PF6− salts release corrosive and toxic HF on contact with acids or moisture under heat. Safe operation requires proper ventilation and sealed storage, often in a desiccator lined with absorbents to trap accidental off-gassing. Waste management involves segregating ionic liquid residues and testing the waste stream for fluoride content before disposal. Official standards like REACH require rigorous documentation. Regular safety training for those working with ionic liquids sets the tone, especially for new arrivals in the lab.
Application Area
In practice, this ionic liquid finds a home in niche applications. Electrochemistry groups load it into test cells for studies in energy storage, where its stability helps when cycling batteries or capacitors at high current. Organic chemists run transition metal catalysis or coupling reactions that struggle in water, using this compound both as solvent and as a reaction medium that doesn't trigger unwanted side reactions. Extraction specialists tackle tough separations—rare earths, precious metals—where conventional solvents fail to dissolve tricky targets. Some renewable energy teams look to this liquid for selective CO2 capture, betting on its tunable gas solubility and low vapor loss. In engineering circles, ionic liquids like 1,3-Dioctylimidazolium Hexafluorophosphate anchor hybrid materials for advanced separation membranes or as lubricants in demanding industrial processes. Some R&D programs even test these liquids as electrolytes in dye-sensitized solar cells for long-term stability.
Research & Development
Funding cycles drive new questions about this compound year after year. Teams at universities and national labs publish data on how slight tweaks to the cation or anion impact solubility, conductivity, and toxicity. Battery researchers keep pushing to understand how ionic liquids can improve charge/discharge rates and lifetime performance. At the interface of engineering and chemistry, people try to design modular synthesis routes so the compound meets new purity standards at bigger scales. Environmental chemists now dig into how these ionic liquids break down in soil or water, aiming to predict real-world impacts. Anyone with experience in translating bench-scale discoveries into pilot runs knows reproducibility drives investment—consistent product means less wasted money and fewer surprises. Open sharing of NMR, MS, and trace impurity data helps everyone avoid costly failures when scaling up.
Toxicity Research
Hazard studies act as the cold shower for every breakthrough. Researchers assess the cytotoxicity of 1,3-Dioctylimidazolium Hexafluorophosphate across cell lines and in aquatic environments, paying special attention to the impact of both the imidazolium cation and the PF6− anion. Acute aquatic toxicity tends to follow a pattern: the longer the alkyl chains, the more risk for bioaccumulation and membrane disruption in living things. Some animal studies show chronic effects at high exposure, highlighting the need for gloves, controlled handling, and proper ventilation. PF6− breaks down slowly, but under strong acid or heat, fluoride release presents a known risk. The community watches closely for new toxicity reports, with journals publishing biomonitoring studies and safe exposure guidelines. Real environmental stewardship means not only fixing leaks and cleaning spills but also building out robust life-cycle analysis that weighs the material’s benefits against risks.
Future Prospects
Looking ahead, the future for 1,3-Dioctylimidazolium Hexafluorophosphate depends on both innovation and responsible management. Open collaboration between industry and academia pushes process improvements and applications without losing sight of safety and ecosystem health. The prospect of using this ionic liquid as a safe, stable electrolyte in next-gen batteries keeps drawing attention. Companies focused on climate solutions see potential for tailored separation processes and direct air capture, betting on the liquid's adaptable solubility. At the same time, safer large-scale synthesis and full transparency around toxicology shape industry standards. Researchers test new routes for recycling ionic liquids, hoping to cut hazardous waste and improve economics. A blend of creativity, technical rigor, and cautious optimism will decide how far this material travels into mainstream use, with every new lab manual and data set written factoring into the honest assessment of its benefits and limitations.
Solvent for Difficult Chemistry
Walking into most advanced chemistry labs, you’ll notice the growth of room-temperature ionic liquids on the shelves. Among them, 1,3-dioctylimidazolium hexafluorophosphate stands out for its stability. Its ability to dissolve a variety of substances makes it a favorite for research and industry. Engineers looking to reduce harsh conditions during syntheses often reach for it. With strong thermal stability and resistance to moisture, this compound lets reactions run at moderate temperatures, which lowers energy bills and increases safety. Research backs up this shift: a 2021 study in Green Chemistry reported a sharp drop in waste output when swapping in this ionic liquid for petroleum-based organic solvents.
Boosting Battery and Supercapacitor Performance
Batteries and supercapacitors rely on materials that move ions efficiently and do not break down under repeated charge and discharge cycles. Here, 1,3-dioctylimidazolium hexafluorophosphate gets genuine attention. Its design lets it carry a current well—without evaporating or corroding electrodes like water-based solutions. In hands-on lab work, I’ve watched how this liquid forms a steady interface with carbon electrodes. Papers published in Electrochimica Acta and Journal of Power Sources have outlined how this ionic liquid supports longer cycle life and improves ionic conductivity in devices that store and release energy quickly.
Advanced Separation Technologies
The chemical sector deals with separating valuable materials from mixtures full of “junk” substances. This often takes place through solvent extraction or more creative separation systems. 1,3-dioctylimidazolium hexafluorophosphate serves as a selective extractant—drawing out metals such as lithium, cobalt, or rare earths from industrial wastes or used devices. At my former employer, a pilot plant staffer told me using this ionic liquid allowed nearly complete recovery of cobalt from shredded batteries, beating out traditional extraction agents. That kind of closed-loop process matters as society leans on electronics and EVs, and recycling turns into a prized resource.
Lubrication and Surface Protection
Some machine and turbine applications grind to a halt when lubricants break down in heat or under high pressure. Ionic liquids have upended this space, and 1,3-dioctylimidazolium hexafluorophosphate stands among the options for extending gear life. The molecules form a protective layer that can limit wear better than classic oils, especially at the small scale. As engineers at a materials conference demonstrated, these surface coatings tackle problems of micro-wear in MEMS (microelectromechanical systems), reducing costly breakdowns and downtime in sensitive equipment.
Potential Headwinds and Opportunities
Wider use of this ionic liquid faces a few snags. Regulatory questions pop up around toxicity and persistence in the environment—folks want results that show these materials break down safely after use. Green chemistry teams continue tweaking the structure for better biodegradability and less risk if spilled. Cost also shapes where it can fit, so scaling up production with cleaner, low-waste methods remains a talking point. Open data and industry–university partnerships could speed progress, building trust with regulators and the public alike.
Looking Forward
Demand for cleaner, safer, and more effective chemicals keeps growing. The experience of innovators filling real-world needs—cutting waste, raising energy efficiency, or protecting vital machines—shows why compounds like 1,3-dioctylimidazolium hexafluorophosphate command attention. Keeping a close eye on safety, cost, and environmental impact will shape where this ionic liquid lands next.
Why Stability Isn’t Just a Lab Concern
Every time someone designs a drug, a cleaner, or even a snack food additive, folks ask about the stability of what they’re making. This isn’t just chemistry for chemistry’s sake—this affects safety, storage, the shelf at the grocery, and the trust we place in products. From my own time working with chemical compounds in quality assurance, the talk about stability always turned practical very quickly. Guaranteeing that a product works the same in July heat or in a six-month-old stock bottle gives peace of mind to everyone from lab technicians to parents.
Breaking Down Chemical Stability
Let’s talk chemical stability in plain language. Heat, light, moisture, and even air can poke holes in a compound’s armor. Once oxygen or water get too friendly with the wrong molecule, the substance can change, sometimes in dangerous or useless ways. For example, vitamin C breaks down quickly in juice left on the counter but holds up much longer when sealed and kept cool. This isn’t trivia; it’s about making sure what you pour in a glass stays good for you.
Some compounds stay rock solid for years. Sodium chloride—the stuff you sprinkle on fries—barely changes with time. Go to something trickier, like nitroglycerin or penicillin, and the picture shifts: temperature swings, exposure to sun, and moisture ruin both. I’ve seen batches go bad just from bad warehouse storage, wasting money and, sometimes, risking safety.
Physical Stability and Daily Experience
Physical stability feels more immediate. Did the pill crumble into powder in the bottle? Is a once-smooth lotion now gritty and weird? A food dye that clumps or a cleaning spray that separates in the bottle—these changes tell you something’s not right. Texture, color, and shape hint at bigger shifts beneath the surface.
I remember a case in a food plant: an ingredient supplier promised a certain shelf life, but clumping became a headache in real kitchens during humid summer months. The problem seemed minor (clumpy powder), but it slowed production, annoyed cooks, and led to waste. A little more attention to physical stability would have saved time and frustration for everyone.
Real-World Solutions and Best Practices
Solving these stability headaches starts in R&D but doesn’t end there. Using proper packaging, storing compounds away from direct sunlight, and educated staff help prevent surprises. In pharma and food industries, stability testing isn’t just a box to check before product launch. Companies run studies in hot, cold, and varying humidity, simulating stuff that’ll happen in trucks, warehouses, and kitchen cabinets.
Regulators expect companies to keep detailed records of how products hold up. Journals and reports give real numbers instead of guesses. Sampling products from actual storage lots keeps folks honest about shelf lives. This practice helps avoid unnecessary recalls, keeps costs down, and, most importantly, keeps people safe.
Moving Forward with Care
Building trustworthy products isn’t just about making something that works the moment it leaves a lab. It’s about making something that lasts—through shipping delays, warehouse mishaps, or simply sitting on a shelf until someone finally grabs it. Paying close attention to both chemical and physical stability means fewer headaches and better results, from the factory floor all the way to the end user. The details matter, and ignoring them usually leads to trouble that could have easily been avoided.
Understanding the Substance
Anyone who’s spent time in a chemistry lab knows that some chemicals demand special respect. 1,3-Dioctylimidazolium hexafluorophosphate stands out for its use as an ionic liquid in electrochemistry and industrial processes. It doesn’t look threatening at first glance, but this is a compound that calls for real care. My first encounter with it came during a solvent conductivity test. It seemed easier to handle than nitric acid—but the reality proved different because of its toxicity potential and reactivity.
Storage Habits: More Than Just Shelf Space
This isn’t the kind of chemical you tuck away with leftover glassware or store in a catch-all drawer. I learned from colleagues that humidity can transform an apparently simple vial into an overflowing mess. 1,3-Dioctylimidazolium hexafluorophosphate absorbs water from the air and breaks down unpredictably. My habit now: always lock it tight in an air-tight container made from glass or compatible plastic and label it clearly. I prefer a desiccator for a little extra protection—silica gel serves as a backup when central drying equipment feels overkill.
Letting sunlight hit the bottle creates real trouble. One summer, a poorly shaded bench turned a safe sample into risky territory—acids formed that shouldn’t have. No one wants to swap a simple task for a hazardous materials drill. So I always park these bottles inside temperature-controlled cabinets. Most labs that care about compliance run around 20-25°C, away from heat sources and UV light.
Handling: Trust Safety Gear and Routine
I’ve watched experts grow careless the longer they work around familiar chemicals. Gloves—nitrile, specifically—come out every time. I never rely on those flimsy disposable types, since splashes and spills may leave hexafluorophosphate ions in skin cracks unnoticed. Splash-proof goggles and a fitted lab coat round out the outfit because even one mistake means a trip to occupational health. My shelves at home never saw a single bottle of exotic salts for just this reason. I like my eyes and skin just as they are.
Pouring and transferring the liquid slow me down. It’s tempting to rush. But spatter and fumes can turn up if you don’t watch your steps. Always work in a chemical fume hood. Ventilation means breathing easy and skipping headaches—literally. Once after a ventilation fan malfunction, lingering odors gave me a migraine and reminded me not to shortcut safety.
Disposal and Emergency Planning
Lab waste bins aren’t meant for ionic liquids like this. Chemical waste teams in reputable institutions usually follow national hazardous waste guidelines. I always double-check disposal procedures before opening any new batch—never after a spill. Hydrolysis, especially with humid air or leaks, can release hydrofluoric acid. My notes from safety meetings stay nearby: calcium gluconate gel is stocked in every first aid kit just in case, after a scare with a similar fluorinated compound years ago.
Small precautions pay off. Every training session emphasizes immediate washing with soap and water for spills, and getting help fast. I watch new lab members closely because it’s easy to break from protocol and let dangerous habits sink in. Surviving a single incident convinced me that routine safety checks and a bit of vigilance save both time and health. This isn’t just policy—it’s personal experience and common sense earned from hours in the lab.
Looking at Risks Beyond the Label
Think back to the last time you picked up a household cleaner or a chemical container. Most people look for directions, but a lot of folks skip over safety instructions or hazard warnings. Years ago, I learned the hard way that even everyday items can cause real harm. I grabbed a strong drain opener without gloves, figuring it wouldn’t be a big deal. My skin reddened and burned for days—something I could have avoided if I’d paid more attention to those warnings.
A surprising number of common products contain ingredients that can cause harm if handled carelessly. Bleach, paint thinners, fertilizers, and even some glues release fumes that irritate eyes and lungs or trigger asthma attacks. National Poison Data System reports show thousands of accidental poisonings and chemical burns each year in homes and workplaces—from people mixing products or skipping the small print.
Reading and Understanding Labels Matters
Regulators don’t put hazard icons and instructions on products for decoration. A strong-smelling cleaner might not seem dangerous, but it can corrode metal or skin and damage lungs if inhaled. Many pesticides and solvents deserve respect—they might help in one problem, but also pose a risk to kids, pets, or the environment when spilled or misused.
Some products look harmless but react with other things nearby. Bleach and ammonia together release toxic chloramine gas. This lesson came from a neighbor whose “spring cleaning” landed her in the ER. Mistakes like this highlight why paying attention is so important.
The Real-World Solution: Take Simple Steps
Nobody needs a lab coat to stay safe with hazardous products. Gloves, safety goggles, or masks keep skin and lungs safer—and they only cost a few bucks at the hardware store. Open some windows or work outside instead of using strong products in a closed bathroom. These habits cut your risk by a lot.
Storing products properly makes a big difference too. I’ve seen kids open cabinets under the sink and start playing with bottles. Products with bold warning labels belong high up, locked away, or somewhere out of reach. Disposing of dangerous leftovers is another step people skip. Pouring paint thinner or old pesticides down the drain puts more than just the plumbing at risk—it can pollute whole water systems. Most towns host special collection days for old household chemicals, making the process pretty simple.
Push for Safer Choices
Manufacturers and lawmakers keep making progress, but there’s room for improvement. Some companies cut back on harsh chemicals, label ingredients clearly, or add child-safety caps. As a consumer, I try to pick these products when I can. Sometimes it costs a bit more, but spending a few extra dollars sounds better than a trip to the doctor.
Questions about product hazards keep coming up, especially as social media spreads lots of guesses and bad advice. Checking reputable sources—like government health sites or poison control centers—beats rumors and hearsay. If you’re ever unsure about a product, call the number on the label or reach out to experts for answers. Being cautious has always paid off in my experience, and it’s a simple thing that protects everyone around you.
Why Purity Matters in Chemistry
Purity drives the entire conversation in chemical sourcing. Traces of impurities can turn a successful experiment into a failed one, compromise product safety, or introduce risks on a factory scale. In labs, even tiny contaminants can affect reaction outcomes and reproducibility. Over the years, I’ve watched scientists stress about an unexpected result, only to trace the issue back to chemical grade or improper storage.
Manufacturers and researchers usually look for purity specifications that guarantee reliable results. For specialty chemicals or pharmaceuticals, 99% and higher purity counts as the gold standard. Analytical methods such as HPLC or GC verify these claims, offering consumers some peace of mind. On product labels, you’ll commonly spot phrases like “ACS Reagent Grade,” “USP Grade,” or simply a percentage like “99.9%.” For industrial use, sometimes technical grades are acceptable, often falling in the 95–98% range, especially when less sensitive applications are in play.
Many people outside the field tend to underestimate how even a half-percent difference in purity can alter outcomes. At the bench, this means cleaner reactions and fewer unknowns. At scale, you’re talking about safer consumer products, less waste, and compliance with ever-tight regulations. I recall a food processor who switched to a slightly purer ingredient and noticed shelf life improvements overnight.
Packaging Options to Match Different Needs
The way chemicals arrive makes a huge difference in workflow and safety. Small-scale research doesn't require drum-sized shipments, and vice versa. Suppliers recognize this, so they fill a wide gap, ranging from tiny bottles to tanker trucks.
Most general-purpose chemicals ship in bottle sizes like 100 grams, 500 grams, or one kilogram, often glass or HDPE to protect contents from air and light. You’ll also find standardized one-liter and five-liter containers for liquids. Walk through a laboratory supply warehouse, and you immediately see shelves decorated with these familiar packages. For regular production, sacks or drums in the 25 to 50 kilogram range are common. The move to stronger, tamper-resistant packaging arose after regulators stepped up scrutiny on workplace accidents and spills. In big industry, bulk packaging rules: you’ll see intermediate bulk containers (IBCs) of 1000 liters, steel drums for solvents, and bulk bags for dry chemicals.
Each package type serves a purpose. Glass stands up to aggressive solvents, plastic resists corrosion, metal offers strength for rugged shipping. Choice of packaging impacts not just storage safety, but also shelf life and cost per unit. In my early career, I worked the back rooms of a university lab, and I never forgot how brittle glass bottles led to frantic clean-ups. These days, improved container materials save everyone a lot of headaches.
Room for Improvement, Safer Outcomes
Despite progress, outdated or improper packaging still causes unnecessary risk—corroded tops, hard-to-read labels, poor seals exposing chemicals to moisture and air. Look to recent industry efforts focused on tamper-proof seals, clear hazard labeling, and eco-friendlier materials. Lessons from past accidents make the case for not cutting corners on packaging and storage. Ask around, and you’ll hear plenty of stories about leaks or mislabeled bottles leading to close calls or ruined work.
Clear standards and transparency from suppliers matter. Companies that disclose rigorous batch testing and traceability gain more trust. In my experience, strong supplier relationships come down to prompt documentation, rapid response to issues, and a willingness to customize packaging or purity grades as needs evolve. No matter the size of the operation, it always comes back to safety and trust in the source.