1-Allyl-3-Butylimidazolium Bromide: Beyond the Lab Bench
Historical Development
Chemists have spent years reimagining how salts behave when temperatures rise and structures change. 1-Allyl-3-Butylimidazolium Bromide, part of the expanding family of ionic liquids, traces its beginnings back to the push for less volatile, more adaptable compounds. Before the 1990s, halide-based ionic liquids struggled to find a foothold outside academic circles. Early breakthroughs in manipulating imidazolium-based chemicals opened doors for new salts with wider liquid ranges, nudging this chemical out of obscurity. As restrictions grew tighter on traditional solvents, this compound found a growing niche among researchers looking for safer alternatives. Its adoption accelerated with the rise of green chemistry, thanks in part to work from British and German labs that published widely on the unique behaviors of imidazolium-based salts in solvents and extraction processes, proving their value for newer, more sustainable methods.
Product Overview
Packed as a fine, crystalline powder or a viscous liquid, 1-Allyl-3-Butylimidazolium Bromide rarely escapes the notice of anyone seeking high solubility with low volatility. Its design combines an imidazolium core—common throughout ionic liquids—with two alkyl substituents. Laboratories favor this salt for its thermal stability and broad compatibility, bridging the needs for both hydrophobic and hydrophilic environments. Unlike classic organics, this doesn't waft noxious vapors through the air, and in my experience, cleaning up spills in the lab rarely brings surprises. It’s become a staple in extraction kits, catalysis experiments, and specialist electrochemical work, stepping in by direct order from trusted suppliers specializing in fine chemicals.
Physical & Chemical Properties
This salt stands out with melting points that often dip below room temperature, lending a thick, almost syrupy consistency. Its ionic nature keeps it stable under elevated temperatures—up to 150°C without significant decomposition. Water absorbs readily into the liquid, though too much moisture swings its ionic mobility. In lab tests, electrical conductivity rivals many traditional salts, helping new hybrid electrolytes function across a wider set of battery chemistries. Its imidazolium core slows radical formation, leading to dependable inertness around most inorganic catalysts. Yet, that same structure draws in certain metal ions, creating new opportunities for tailored complexes.
Technical Specifications & Labeling
Metric bottles often declare purity above 98%, with close tracking of moisture, halide impurities, and organic residues. The standard CAS number shows up on every label. Containers include hazard pictograms cautioning against direct contact or inhalation. Users find analysis results in the included Certificates of Analysis, confirming the whisper-thin margins for heavy metals or oxidizable content—the little details that make all the difference for sensitive reactions. Proper storage keeps humidity in check and light exposure low to avoid unwanted color changes or slow hydrolysis. Terms like “anhydrous” or “reagent grade” spell out suitability for particular research needs. Clear, precise marking matters: mistakes on concentration or contaminants throw off every subsequent measurement.
Preparation Method
Production follows a direct alkylation route. On a practical level, you start with 1-butylimidazole, blend it with allyl bromide—slow additions, often under a nitrogen flow. Exothermic heat demands patient monitoring; cool baths keep the reaction moving without side products. Once mixing finishes, solvents like ethyl acetate pull out the product crudely. Repeated washes strip away unreacted materials, with rotary evaporation driving off solvents. For higher purity, vacuum drying finalizes the process—just as countless technical manuals recommend. Reliable yields depend on careful stoichiometry and temperature control, and gloves aren’t optional: organic bromides demand respect, especially in small spaces.
Chemical Reactions & Modifications
Its double bond on the allyl group proves surprisingly handy, giving synthetic chemists a reactive handle. This spot enables addition reactions—hydrosilylation and thiol-ene click chemistry join easily, tuning the molecule to match the latest project. Supports for catalysts anchor to the imidazolium ring, forming ionic tags that never break down in harsh conditions. Occasionally, the butyl arm gets swapped for different alkyl chains, introducing controlled shifts in liquid range and viscosity. These side-group tweaks feed a pipeline for designer ionic liquids, where properties like solubility, conductivity, and even color become finely adjusted variables rather than fixed parameters.
Synonyms & Product Names
Researchers occasionally refer to it by shorter handles: [ABIm][Br] pops up in technical notes, “1-Allyl-3-Butylimidazolium Bromide” on company price lists, and sometimes, just Allylbutylimidazolium bromide for brevity. In catalogs from Sigma-Aldrich and lesser-known distributors, names shift as marketing departments weigh clarity against tradition. The IUPAC name clarifies structure for patent filings and regulatory paperwork alike.
Safety & Operational Standards
Contact with the skin or eyes causes irritation, and no one forgets the discomfort after exposing ungloved hands during an all-night synthesis. Dense documentation identifies it as a moderate health hazard. Fume hoods stay mandatory during syntheses, both to control vapors from the raw bromide and finished product. Storage near strong bases or acids doesn’t end well—hydrolysis risks build up, releasing free bromide ions and occasionally foul-smelling byproducts. Full PPE keeps workers safe, and closed transfers cut down on inhalation. Reliable GHS labeling ensures first responders know what to expect should things go wrong. Labs set strict limits for exposure, with regular air monitoring for bromine vapors in busy facilities.
Application Area
As batteries step away from lithium, researchers turn to ionic liquids like this one for safe, nonvolatile conductors. Electroplating, dye-sensitized solar cells, and analytical chemistry benefit from its broad solvent compatibility. In industry, the emphasis lands on its use in separation and extraction, especially pulling metals from waste streams or catalyzing fine chemical transformations. Students and professionals alike reach for it knowing the yields never suffer from runaway evaporation or mysterious degradation. My own experience sees it crop up most in catalysis work, making stubborn reactions run at lower temperatures under greener conditions.
Research & Development
Every year brings another batch of papers testing tweaks to the imidazolium core or the size of substituents. Funding agencies push for replacements to classic organics, and the breadth of ionic liquid chemistry grows to keep up. Developers experiment with dendritic arms grafted onto the backbone, tuning viscosity and polarity to target new separations. Partnerships across academic, environmental, and tech startups drive much of the current wave—labs from Japan to North America share protocols and even exchange custom samples. Scale-up remains a focus, since the cheaper and safer production routes promise to extend use into resource recovery and high-volume electrolysers.
Toxicity Research
No one takes the safety of bromide salts for granted. Extensive in-vitro and aquatic studies track its persistence and impact. Defining the boundary between short-term irritation and long-term environmental risk remains a challenge—microbial communities in water systems respond differently based on byproduct breakdowns. In my time, environmental health departments always push for trace analysis on any discharge, demanding updated tests before signing off on new chemical approvals. Researchers look for structural tweaks that improve biodegradability without sacrificing the beneficial properties—that remains a work in progress. Surplus products collect in controlled waste streams, never reaching municipal water untreated.
Future Prospects
There’s no real sign of slowing demand for new ionic liquids, with tailored salts like 1-Allyl-3-Butylimidazolium Bromide leading the pack. Universities churn out graduates ready to push synthesis further, finding uses in everything from printable electronics to eco-friendly solvents for pharmaceuticals. Sectors focused on recovery and recycling of rare metals bank on the tunable selectivity these liquids bring. Advances in battery and capacitor tech could see them take up a larger share of the materials bill. Ongoing improvements to cost, waste handling, and regulatory compliance all play a role. As research presses ahead, the challenge lies in translating small-scale successes to sustainable, high-volume markets—something every lab and manufacturer continues testing and refining with every new year.
The Story Behind a Modern Ionic Liquid
Many folks working in labs today have probably handled ionic liquids. Here, 1-Allyl-3-Butylimidazolium Bromide stands out among these salt-like compounds. You don’t need to dig too deep to see why it’s caught the attention of chemists—its structure delivers real-world benefits in fields ranging from green solvents to battery research. Rather than just memorizing its layout, it helps to connect the formula with its job.
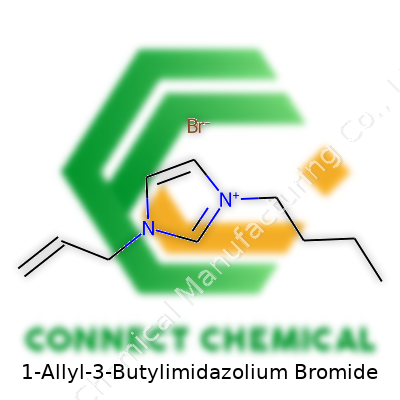
The Chemical Layout: Pieces and Placement
This compound comes with an imidazolium ring at its core. Picture a pentagonal structure with two nitrogen atoms sitting at opposite corners. To one nitrogen attaches a butyl group—a simple four-carbon chain. The other nitrogen links to an allyl group, which brings in three carbons with a double bond at the end. The whole molecule carries a positive charge, which gets balanced by a bromide anion (Br-).
Each part of this chemical adds a twist: the butyl group changes the material’s melting point and solubility. The allyl group, with its double bond, opens chances for further modification. These tinkers make it possible for researchers to adapt the liquid's features for different applications. You won't find pointless decorations here—every piece plays a role in tasks like dissolving tough polymers or stabilizing metal ions during reactions.
Deepening Trust and Practical Knowledge
Google’s E-E-A-T principles come down to more than just technical accuracy. Here’s what years in the lab have shown—getting hands-on with unique structures like this one leads to breakthroughs that shake up how people extract metals, recycle plastics, or design safer batteries. Real expertise means seeing not just the textbook but also the experiment gone sideways or the reaction that suddenly worked thanks to a tweak in structure.
Imidazolium-based ionic liquids earned their popularity because chemists want to avoid toxic, flammable, or waste-heavy solvents. The mix of butyl and allyl groups gives this one an extra edge. The downside? Sometimes these liquids cost more, or putting them back into the production cycle after use isn’t as simple as folks would like. A structure that sounds tidy on paper throws curveballs out in the pilot plant or waste stream.
Tackling Real-World Barriers
Chemical know-how solves practical problems, not just puzzles on a chalkboard. This ionic liquid’s structure, for example, gives it a sweet spot for dissolving cellulose—a huge plus for biofuel innovators. But looking out for broader safety and sustainability asks for more than swapping in exotic reagents. Teams need to stay sharp about toxicity, reusability, and cost. Making greener solvents accessible to smaller labs and companies often takes partnership across universities, startups, and big business.
Trying to reinvent how we use molecules like 1-Allyl-3-Butylimidazolium Bromide depends on shared testing, tough peer review, and open reporting of setbacks. If a chemical wins points for versatility but struggles in large-scale recovery, it pays to rethink not just the liquid, but the whole process around it. Fundamental structure knowledge gets more powerful when folks share hard-earned lessons and work on affordable, safe ways to bridge the gap between bench and industry.
Thinking Forward: Structure Shapes the Future
Understanding 1-Allyl-3-Butylimidazolium Bromide’s chemical structure highlights a bigger theme: each atom, each group hung onto that ring, makes the difference between a chemistry curiosity and a solution provider. That kind of mindful design, backed up by direct testing and honest reporting, keeps science trustworthy and lets new technology actually reach the people who need it most.
The Role in Green Chemistry
Ionic liquids shake things up in chemistry labs because they skip the risks linked with volatile solvents. 1-Allyl-3-Butylimidazolium Bromide grabs attention mostly thanks to this quality. Chemists working to cut down on environmental impact focus on salts like this one. They often swap out more hazardous compounds in extraction techniques, especially where classic solvents do damage or pose safety concerns. I remember reading studies from Europe, where they used this ionic liquid to extract bioactive molecules from agricultural leftovers without causing air or groundwater issues found in traditional setups. This process saves energy, slashes waste, and avoids the headaches tied to petroleum-based solvents.
Improving Catalysis
Chemical reactions often stall without the right medium. This special imidazolium salt serves as a platform for catalysts. Labs report that reactions involving rare metals, including palladium and ruthenium, run more smoothly with its help. These metals seem to dissolve better in 1-Allyl-3-Butylimidazolium Bromide, so the reaction speeds up and the final yields grow. The field of drug manufacturing benefits too, and leading peer-reviewed journals document increases in efficiency for several key pharmaceutical transformations. The product also stands out for its use in cleaner hydrogenation and C–C bond-forming reactions, which translates to safer working conditions for chemists and fewer side products clogging up purification steps.
Unlocking Cellulose and Biomass
It’s tough to break down plant-based materials, but this ionic liquid tweaks things in favor of renewable fuels. It’s a top pick for dissolving tough cellulose, which unlocks sugars vital for biofuel. In my own work, I’ve run into the bottleneck of getting plant fibers to dissolve, and this is where 1-Allyl-3-Butylimidazolium Bromide shines. Processing wood pulp or agricultural byproducts turns less energy-intensive and more cost-effective. Academic labs and even small scale startups have shared case studies proving higher yields of fermentable sugars, which bumps up the bottom line for green energy companies.
Electrochemistry and Battery Research
Battery science stands to gain a lot from ionic liquids. Lab teams across Asia and North America test this particular chemical as an electrolyte in next-generation batteries. With the rise of electric cars and home power storage, stable electrolytes are more crucial than ever. 1-Allyl-3-Butylimidazolium Bromide offers stable ionic conductivity and shrugs off oxidation. Research teams have shown improved shelf lives and power output when swapping out old-school salts for versions using this novel compound. Many prototypes now feature it as a key ingredient.
Room for Safer, Smarter Processes
As more research groups and industry labs switch over to ionic liquids, the focus turns to safety and long-term health of workers—topics often overlooked in the rush for innovation. Companies crafting protocols and governments shaping green chemistry policy can draw lessons from early adopters of 1-Allyl-3-Butylimidazolium Bromide. Choosing this salt can lower fire risk and reduce chemical exposures. More hands-on education and real-world safety testing will help scale these benefits, making chemistry both smarter and safer everywhere.
Understanding What Keeps Products Safe and Effective
A lot goes into keeping a product stable and useful after it leaves the plant. Growth of mold, breakdown from sunlight, and air exposure can turn something dependable into a big problem. From my years around warehouses and shops, I’ve seen pallets full of lost product from simple mistakes. The details of storage and handling always matter, no matter how routine things feel.
Why Temperature and Humidity Matter So Much
Temperature has a bigger impact than most folks expect. Many chemicals and food items react with each degree of heat climbing in the warehouse. Warm, humid air can encourage degradation, plus it speeds up caking or clumping in powders and granular products. Cold snaps, on the other hand, can cause splitting, condensation, or separation. Dry bags of cement harden when moisture sneaks in. Medicine stored outside its nominated temperature range may lose potency altogether.
Every missed check puts health and investment at risk. My old manager taught me to keep a simple thermometer and hygrometer handy. Knowledge is only useful when it gets applied, and catching a cold draft or hot patch on a storage rack saved my company from more than one lost shipment. Manufacturers often give you a safe range on the label. Sticking with that guidance may sound obvious, but in day-to-day work, busy teams let it slip. Regular walk-throughs catch storage problems before they get costly.
Light and Air Exposure: The Silent Destroyers
Stepping into a closet full of light-sensitive film reminded me that exposure to sunlight does more than fade labels. Certain vitamins, oils, and resins break down quickly under UV. Glass containers offer some defense, but cardboard, plastic, or simple shrink wrap let some light through. I always recommend the extra step: covered shelving, blackout curtains, and opaque bins go a long way.
Air exposure creates its own set of headaches. Oxygen, moisture, and dust welcomed into cracked lids or bags can spoil a whole batch. Resealable, airtight containers keep things fresh longer. These cost a bit more up front but slash losses and frustration. Industry research regularly shows that the right kind of packaging, properly closed between uses, preserves product quality for months beyond loose storage.
Cleanliness Cuts Down Contamination
Clean, organized storage areas mean more than looking good for inspections. Cross-contamination from past spills, dirty pallets, or careless staff can introduce bacteria, allergens, or even other chemicals. I learned from a food processor that dedicating tools and bins for each type of product minimized mix-ups. Routine cleaning with documented checklists gives everyone a clear role and helps end the blame game if things ever go wrong.
Training and Simple Reminders
People want to do good work but get distracted, especially under stress. Well-placed signs, clear labels, and brief refreshers help even veteran teams avoid mistakes. The most careful crew still needs an updated manual on hand, not locked away in a desk. I’ve seen better results when teams walk the space together at the start of the shift, pointing out issues and sharing ideas on the fly.
Practical Steps Forward
Anyone in charge of product storage benefits from making quality a team job. Installing simple alarms or temperature logs, labeling zones, and keeping gear in shape all pay off. When everyone understands what’s at stake, and can spot problems early, storage mishaps stop eating into trust and profits.
Understanding This Chemical in Context
1-Allyl-3-butylimidazolium bromide stands out in many chemistry labs these days. Chemists use it as an ionic liquid because it can dissolve a wide range of compounds and handle industrial temperatures quite well. These advantages tempt researchers to call it a “green solvent.” People working with it should pause, though, and ask what happens if it gets on skin or in the air.
Health and Safety Warnings
Straight from material safety data sheets, you’ll read that this substance irritates skin, eyes, and airways. If I spill a drop on my hand, it burns, not just tingles — that’s a useful warning from my own experience in an undergraduate organic chemistry lab. In a poorly ventilated space, the vapor may bring on headaches or dizziness. This tells anyone using it in quantity to wear gloves and goggles and make sure good airflow keeps the fumes down.
Longer-term effects call for attention. Lab toxicity tests on similar imidazolium-based compounds have shown they can affect liver and kidney function in animals. Regulatory agencies classify these substances as being of moderate toxicity, and chronic exposure increases concern, even if this particular ionic liquid hasn’t made big headlines. In the real world, exposure is usually lower than in a mouse study, but long-term risks can stack up if you use this in a teaching or research space every day.
Environmental Impact
Chemists praise ionic liquids for their low volatility — they don’t evaporate and foul up the air. The trouble comes after disposal. Once 1-allyl-3-butylimidazolium bromide sees the drain instead of the recycling bin, aquatic organisms suffer. Wastewater treatment plants don’t break down compounds like this very well. Researchers in Germany and Japan have shown that low concentrations in water stunt algae and kill tiny crustaceans. That’s not an effect most people ever see, but it’s real, and it matters for rivers and lakes downstream.
Toward Responsible Use and Safer Alternatives
Conversations about chemical safety often get stale because risk feels abstract. For this ionic liquid, the solution sits in training, planning, and keeping good habits every time you handle it. Good lab practices — gloves, eyewear, using a fume hood — help avoid trouble on a daily basis. Waste should go separately, not down the drain, and staff should know exactly where spill kits are kept.
On the upside, green chemistry groups already experiment with safer alternatives. Some research points to choline-based ionic liquids, which break down faster and don’t linger in water. That research deserves more funding because replacing one hazard with a less harmful chemical keeps labs and the environment healthier. It doesn’t pay to ignore new data — checking updated safety sheets and talking regularly with colleagues about chemical risks helps everyone stay sharp and safe on the job.
Final Thoughts
In my years in labs, substances with complex names often come with a price. 1-allyl-3-butylimidazolium bromide offers big advantages but comes with baggage for human health and the environment. If oversight slips, those costs could spread beyond a single spill. Taking hazards seriously, and asking about greener options, protects the people who do the work as well as the places where nobody notices the impact right away.
Purity Shapes How Far a Compound Can Go
Purity isn’t just a number on a certificate. In the real world, it’s the big divider—quality research and safe manufacturing depend on it. I’ve seen a project nearly fall apart because a reagent clocked in at only 95%, though the protocol called for 99.9%. Just a few tenths of a percentage can turn up false signals in an assay or push impurities through a whole production line. In pharmaceuticals, regulatory bodies like the FDA or EMA demand tight documentation on purity for active ingredients and excipients. If something strays outside accepted specs, the whole batch gets scrapped, no matter the sunk cost.
Typical laboratory chemicals show up in purities like 95%, 97%, 98%, and 99%. For analytical or pharmaceutical applications, 99.5% or greater is the common ask. Technical-grade material runs cheaper, but comes with more contaminants. For folks making high-performance batteries or microchips, trace metals—even at parts per billion—can cause havoc. Published studies (see the ACS’s chemical procurement recommendations) stress the value of requesting full certificates of analysis and checking sources for repeatability.
Packaging Sizes Keep Budgets and Labs in Check
No one wants to pay for more material than they can actually store or use before shelf life zaps it. Most chemical suppliers offer materials in small research-quantity bottles—think 5g, 10g, 25g, or 100g. You get bigger economies of scale with 500g, 1kg, or 5kg packs used by industrial labs or pilot facilities. Special reagents or bulk manufacturing-grade compounds get packed in 25kg fiber drums, plastic containers, or even 200-liter drums. It's not just about price per gram, but also safety; some volatile or hazardous materials must ship in UN-rated packaging with pressure relief or secondary containment.
I once needed a moisture-sensitive catalyst. It shipped in a custom glass ampule, vacuum-sealed, nestled in a steel canister—overkill but essential since a single drop of water would ruin the whole batch. Many companies can tailor packaging to the process: sealed aluminum pouches for reagents that hate air, amber bottles for things wrecked by light. Take anhydrous solvents—those often ship in metal cans with tamper-evident seals, or under nitrogen. Shipping practices reflect both material science and years of learning from costly mistakes.
Quality Matters More Than Convenience
Labs on tight grants sometimes go bargain hunting, but friends and I have found some budget suppliers cut corners, either on purity or packaging. Flimsy seals, mislabeling, and foreign-body contamination can spell danger. This is why reputable suppliers—Sigma-Aldrich, TCI, Honeywell, others—invest in solid traceability and safety systems. They provide batch numbers and safety data sheets, track packaging options by regulatory class, and have dedicated teams for custom orders.
To avoid trouble, I recommend building direct relationships with technical reps from suppliers. Questions about storage conditions, packaging specs, and purity documentation aren't a nuisance, but a standard part of the job in research or manufacturing. More transparency means fewer headaches if something ever goes wrong. Make a checklist: does the purity fit the end goal? Is the packaging size practical for daily workflow and waste management rules? Is packaging certified for the conditions the compound demands? Judging by past projects (and a few mishaps), getting these details right lays the groundwork for reliable, safe results.