1-Allyl-3-Butylimidazolium Hexafluorophosphate: A Close Look at a Modern Ionic Liquid
Historical Development
1-Allyl-3-butylimidazolium hexafluorophosphate, often recognized by chemists as a part of the ionic liquid family, pulled ahead in laboratory circles in the late 1990s as research on environmentally friendlier solvents gained traction. Traditional organic solvents, like acetone or dichloromethane, brought cleanup headaches and safety concerns that collected like bad habits over years. Green chemistry researchers explored alternatives, searching for chemicals that could sidestep volatility and minimize hazardous waste. Ionic liquids, especially those based on imidazolium cations and paired with anions like hexafluorophosphate, looked promising. By early 2000s, organizations focused on energy storage, catalysis, and electrochemical devices had zeroed in on these compounds. The story here highlights a real shift—industrial chemists realized ionic liquids offered new ways to tackle problems rigid organic solvents could never master.
Product Overview
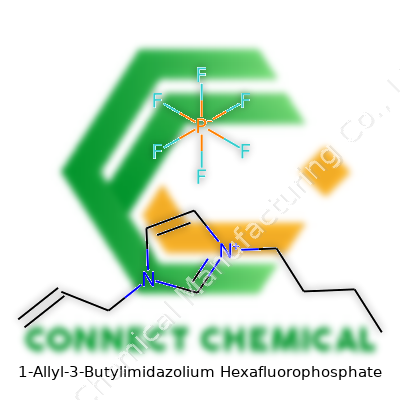
The structure of 1-allyl-3-butylimidazolium hexafluorophosphate features an imidazolium ring with an allyl and a butyl chain branching from the nitrogen atoms, paired with the PF6– anion. It doesn’t carry the pungent odor often tied to volatile chemicals on the bench, and you won’t see its vapor fogging up fume hoods—this liquid stays in the flask, with almost no vapor pressure under standard conditions. That makes it attractive for labs aiming to reduce solvent exposure. Many researchers have started with commercial samples, but homegrown synthesis remains common in academic labs chasing custom cation or anion combinations. In my early lab days, handling samples of ionic liquids like this one made constant glove changes less necessary; no skin burns or eyes watering from fumes.
Physical & Chemical Properties
You can recognize 1-allyl-3-butylimidazolium hexafluorophosphate as a clear or faintly yellowish liquid at room temperature. It pours with a syrupy viscosity reminiscent of light honey, which often surprises people who expect something as runny as water. With a melting point below ambient lab temperature and a boiling point that basically doesn’t exist under normal atmospheric pressure, thermal stability makes the substance useful in settings where traditional solvents would evaporate away. Its density typically falls just above 1 g/cm3 at 25 °C, so small spills spread but don’t seep too deeply. The hexafluorophosphate anion lends strong resistance to hydrolysis, giving this ionic liquid remarkable shelf life in closed containers. It dissolves both organic and inorganic species, making it popular for specialty extractions and two-phase reaction systems.
Technical Specifications & Labeling
Manufacturers assign CAS number 171058-17-6 to this compound, with common labeling that notes moisture sensitivity, and recommends storage in airtight bottles, dry places, and away from strong acids or bases. Purity levels up to 99% ensure reliable performance in high-precision applications—for example, catalysis and analytical chemistry. Labels often highlight conductivity and water content, since excess moisture can degrade performance or skew research data. The product sometimes ships under inert gas or with desiccants for long-term stability. From my own work, careless storage lets ambient water creep in, leading to cloudiness and unpredictable chemistry—minor mistakes that can sabotage weeks of experiments.
Preparation Method
Synthesis typically kicks off by alkylating imidazole with allyl halide to form 1-allylimidazole. This intermediate then reacts with butyl bromide in an aprotic solvent such as acetonitrile, leading to 1-allyl-3-butylimidazolium bromide. A straightforward metathesis reaction exchanges the bromide for hexafluorophosphate, carried out by combining with a source such as potassium hexafluorophosphate in water or polar organic solvents. After phase separation, thorough washing, and careful vacuum drying, you’re left with the pure ionic liquid. Getting the washing and drying right matters; even small contamination from side products or water can undermine its role in catalysis or affect conductivity in electrochemical studies. I learned that lesson the hard way when an underwashed sample threw off an entire set of electrochemical test results.
Chemical Reactions & Modifications
The double bond in the allyl group opens doors to functionalization you won’t see with simpler imidazolium salts. Researchers have carried out additions, such as hydrosilylation, or used the reactive site for crosslinking and polymerization. Most notably, exchanging the anion—in this case, hexafluorophosphate—for alternatives such as bis(trifluoromethylsulfonyl)imide (NTf2–) tunes solubility, hydrophobicity, and performance in different contexts. In practical lab work, I’ve seen people anchor catalysts on the cation scaffold, then tune the anion to match whatever task sits at hand, such as CO2 absorption or enzyme stabilization.
Synonyms & Product Names
Marketed under various names like [AllylButylIm][PF6], ABI-PF6, or its more systematic name, 1-allyl-3-butyl-1H-imidazol-3-ium hexafluorophosphate, the compound crops up in catalogs from multiple specialty chemical suppliers. Keeping track of the synonyms avoids confusion, especially when digging through papers or patents that each may favor a different naming approach.
Safety & Operational Standards
Despite its low volatility, 1-allyl-3-butylimidazolium hexafluorophosphate needs careful handling. Hexafluorophosphate-containing compounds aren’t risk-free; under acidic or high-temperature conditions, there’s a shot at forming toxic hydrogen fluoride. Gloves and goggles come standard in the lab, and working with airtight barriers keeps exposure to a minimum. Standard waste disposal involves specialized containers, since landfill or sink disposal carries environmental risks. Lab colleagues and I have always used chemical fume hoods with these substances even though they bypass the noxious fumes—safety protocols exist for a reason.
Application Area
This ionic liquid found a niche in electrochemistry, outshining many traditional solvents or electrolytes in batteries, supercapacitors, and fuel cells. Its ability to dissolve both salts and organic molecules widens its footprint in organic synthesis, especially as a reaction medium that can stabilize transition metal catalysts or aid selectivity in cross-coupling reactions. Extraction processes also take advantage of its unique solubility properties—efficiently separating metallic ions, extracting valuable or toxic organics, or remediating contaminated water. Labs focused on carbon capture have experiment with this family of ionic liquids to absorb and sequester greenhouse gasses. Drawing on personal experience, I’ve watched researchers push the envelope, running multi-step syntheses that would fail outright in old-school organic solvents.
Research & Development
R&D teams worldwide treat 1-allyl-3-butylimidazolium hexafluorophosphate as more than just another solvent. They push into new spaces—developing polymer-compatible materials, designing stimuli-responsive gels, or embedding the compound in sensor arrays. Collaborations with computational chemists have mapped the electronic structure to explain how this class of chemicals helps dissolve rare metals or stabilize proteins. Lately, pharmaceutical research eyes this ionic liquid for its ability to mediate challenging coupling reactions, chipping away at some persistent bottlenecks in medicinal chemistry. Working with graduate students over the years, I’ve seen this compound become a familiar testbed for creative experiments, underscoring how real-world progress rarely happens alone or overnight.
Toxicity Research
Safety remains closely studied. Hexafluorophosphate-based ionic liquids, although less volatile than common solvents, draw scrutiny for aquatic toxicity and environmental persistence. Research points to moderate bioaccumulation and possible hazards to fish and aquatic invertebrates. Chronic exposure data in humans doesn’t stack up to traditional solvents yet, but regulatory agencies recommend tight controls, especially on waste disposal and accidental spills. In teaching labs, I’ve always emphasized containment and careful cleaning for all ionic liquids—nobody wants to gamble with unknown or underestimated health risks, no matter how advanced the technology feels.
Future Prospects
Looking forward, 1-allyl-3-butylimidazolium hexafluorophosphate stands poised for broader roles as scientists keep chasing greener chemistry and more specialized applications. Growing demand for efficient energy storage, cleaner synthesis, and pollution control creates strong incentive to improve production, cut environmental impact, and expand safety research. More research into tuning cation or anion pairs, or into breaking down ionic liquids after use, could answer many lingering environmental questions. Watching the trajectory of green solvents, one thing stands out: compounds that adapt to stricter regulations and deliver robust performance won’t stay niche for long. As knowledge spreads, so should best practices—for both the breakthroughs and the cautionary tales.
A Look at the Chemical’s Role in Modern Industry
Chemistry sometimes feels like it’s happening far away, tucked into labs or factories, but the compounds people discuss there often touch real problems and innovations. 1-Allyl-3-Butylimidazolium Hexafluorophosphate—long name, yes—is one of those ionic liquids carving out an essential space in the world of chemistry. Its practical uses stretch from making better batteries to supporting green chemistry. People often overlook how a single chemical can shake up a whole process.
Helping Out in Electrochemistry and Batteries
One application that stands out involves energy storage. The switch to electric vehicles and renewable energy demands better batteries, and researchers experiment with new stuff all the time. Developers add this imidazolium-based liquid to lithium-ion batteries, chasing improved stability and longer lifespan. It does a good job acting as an electrolyte, and doesn’t catch fire as easily as some organic solvents used in the past. Safety and efficiency matter a lot in energy. In fact, some recent studies showed that batteries using these fluids can work over a wider range of temperatures, and the batteries stand up to charge cycles without breaking down quickly. Companies and labs got excited about using liquids like this for supercapacitors, too, hoping for faster charging and longer lives.
Pushing Green Chemistry Forward
Lab workers use 1-Allyl-3-Butylimidazolium Hexafluorophosphate as a solvent for organic reactions. Solving chemical problems often means dissolving other chemicals, and many classic solvents are harsh on people and the planet. This ionic liquid doesn’t evaporate quickly and doesn’t release toxic fumes. People keep looking for safer ways to build medicines or specialty chemicals, and switching to less hazardous solvents gets attention from both researchers and regulators. Teams trying to cut industrial waste and do things cleaner see this compound as an option in processes like alkylation, Diels–Alder reactions, and extraction steps. In my chemistry days, swapping out toxic solvents for safer options made the whole job more pleasant—and it pushed the industry one step forward.
Role in Material Science and Metal Processing
There’s always demand for cleaner ways to process metals. Electroplating, for example, leaves a trail of harsh waste and forces engineers to juggle toxic chemistry. Using this ionic liquid as an electrolyte lets companies coat metals without toxic byproducts. In material science, specialists add these liquids to help create nanoparticles or separate metals more efficiently. People working with rare earth elements or precious metals, especially, look for ways to keep processes compact and sustainable. Recent journal articles show successful use in metal extraction—getting more out of mined ore with less leftover waste.
Challenges and What Could Come Next
Chemistry isn’t free from hurdles. Many of these ionic liquids—including this hexafluorophosphate variety—cost a fair bit to produce, and disposing of them still raises questions about environmental impact. Researchers keep tweaking the structure to reduce toxicity even further or use more abundant starting materials. The push for more affordable versions comes from universities and private sector labs alike, especially in energy and manufacturing circles. Every step toward safer, more robust materials gives both industry and researchers more breathing room to innovate without leaving a bigger mess behind.
Looking Beyond the Label
People often pick up a product and glance at its expiration date, but few pause to consider what that date really stands for. Chemical stability dictates how long something remains unchanged and safe to use. Shelf life pulls together those details and sets a clear limit. Products in medicine cabinets and under-sink cupboards rely on chemical bonds holding firm until called upon. That’s easy to forget until an old cough syrup delivers zero relief, or household bleach falls short and leaves a surface unclean.
What Shapes Chemical Stability?
Any product’s stability ties back to its ingredients and packaging. Take food preservatives or active drug ingredients—light, air, heat, and even moisture kick off slow reactions. Sometimes, these reactions spoil the product or create new compounds that cause unexpected side effects. I’ve seen this firsthand in the pharmacy setting. Some over-the-counter painkillers can lose their punch months before their expiration if left in a steamy bathroom. The 1950s saw tragic cases where a breakdown in a liquid antibiotic caused toxicity. Cases like that led to strict safety standards in the industry, and today, hospitals and consumers demand reassurances.
The FDA and industry researchers put products to the test, subjecting them to high temperatures and humidity. These “stress tests” reveal weak spots and show how rapidly active ingredients or preservatives degrade. I remember reviewing lab results that showed some vitamins shrink by half in potency after a year of improper storage—a real wakeup call for companies and families who want to avoid wasting money and risking safety.
Packing and Practice Make All the Difference
The box or bottle carries a lot of the weight. Glass blocks out air and moisture better than plastic in most cases. Some drugs, like nitroglycerin tablets, lose power fast in standard pill bottles. I’ve had elderly patients tell me their new refills “don’t work,” only to learn those tablets had sat exposed on a sunny windowsill. That kind of mistake can become a life-or-death issue. Even food items like spices lose color and flavor quickly if left uncapped or near the stove. Once packaging fails, the clock runs out faster.
Facing the Future: Solutions for Better Shelf Life
Better education would help people fully understand shelf life instructions. Schools teach food safety, but few offer details on medicine or home product stability. Clear graphics on packages could spell out “store in a cool, dry place” and maybe even show what happens if you don’t. Companies researching new materials keep pushing for tougher, more airtight packaging. Sealed blister packs for pills and vacuum-sealed food jars already offer much longer shelf life compared to old paper wraps or simple twist caps.
Real transparency matters. Too many companies tuck stability data behind technical jargon or in fine print. Consumers trust products more when companies plainly state what to expect, how best to store items, and what changes signal a problem. Supporting this kind of open information and research, guided by standards from groups like the FDA and WHO, can make a big difference in everyday safety and value.
Bringing It Home
Chemical stability may sound like a dry technical word, but it touches almost every shelf at home and work. A little attention to handling and storage, from the right bottle to the right shelf, protects health and wallet alike. Experts and everyday people working together keep products stronger for longer, and that’s always worth the effort.
Why Proper Safety Matters Every Day
Too many people in workplaces or at home think they’ll never face real danger from a chemical. Maybe you remember your own first time reading a safety sheet—feeling confused or even skeptical about how big the risks actually are. It can look like overkill when you haven’t seen an accident. Though, stories pile up: someone suffered burns from a splash, another person inhaled a vapor cloud and ended up coughing for hours, a student mixed two things in a lab and sent classmates to the nurse. These aren’t rare events. Facts show that accidents in labs, factories, or even garages lead to thousands of emergency room visits each year. That’s a wake-up call. Personal protection and equipment makes a difference every day—not just when the danger “feels” obvious.
Essential Steps for Safe Handling
Gloves and goggles top my list, and I never skip them if there’s any risk, even a small one. I’ve seen folks refuse gloves because “it’s just a splash risk,” then spend their afternoon flushing their hands under a cold tap. Nitrile, neoprene, or other appropriate gloves keep skin safe. Eye protection keeps splashes and sudden sprays from turning a train of thought into panic.Ventilation changes the whole game. I’ve spent time in labs where even a small spill filled the air with fumes, setting off alarms and causing headaches for everyone nearby. A good fume hood, exhaust fan, or outdoor workspace reduces those risks. Breathing protection isn’t just for those working with obvious toxins. Dust masks or respirators save your lungs with powders or volatile liquids.
Know the Compound—Read, Learn, Prepare
Every time a new bottle appears on a bench or shelf, take ten minutes and read the label and Safety Data Sheet. Not every compound looks dangerous—some are colorless or odorless. Hidden hazards, such as slow-release toxic gases or delayed skin reactions, don’t announce themselves. Look for information about how a substance reacts with water, air, or common organic solvents. Some reagents burst into flame or create toxic gases with just a drop of water. Others degrade plastics or metal tools.Storage rules aren’t there for decoration. Mixing acids and bases, or acids with organics, leads to heat and gas. Segregating bottles according to chemical class keeps things simple and prevents silent disasters inside cabinets.
Plan for Accidents, Not Just Routine
Spill kits and eyewash stations don’t collect dust for long. I once worked in a lab where someone dismissed the need for an emergency shower, until hot alkali spilled across a forearm. Quick reaction saved the skin, and it came down to having clean water and neutralizers in arm’s reach. Regular training and drills pay off—panic slows down thinking, but muscle memory gets you to the nearest rinse station or ventilation switch.Label everything. Don’t rely on memory or half-torn masking tape. Clear labeling stops confusion between solvents and acids, or plain water and a peroxide solution. It only takes one bad mixup to trigger a hospital trip or a costly decontamination.
Culture of Safety Leaves No Room for Shortcuts
Safety habits come from people caring for each other. Set the example for your coworkers, students, or kids: gear up, read instructions, clean up as you go, and never rush. Chemicals play an invisible but real waiting game. If you make safety normal, accidents drop fast. Peer-reviewed studies confirm that workplaces with steady safety training and clear personal responsibility see far fewer injuries and illnesses.There’s no heroism in skipping protection; only an increased likelihood of injury down the line. Show respect for the chemicals and for those working nearby by putting safety first, every time.
Melting Point: More Than a Number
Growing up, I once watched a friend try to make a candle just by pouring melted paraffin into a cup of cold water. The wax froze instantly, crumbling into useless shards. It taught me early that melting point is much more than a boring note in a science book. The melting point tells us at what temperature something changes from solid to liquid. This isn’t just lab work. Chocolate with a melting point just below body temperature melts in your mouth but not in your hand. A high melting point keeps asphalt from becoming a gooey mess in the summer. These aren't just conveniences—they keep food enjoyable and city roads drivable.
The data speaks volumes. Chemists rely on melting points to check purity. Impure substances melt at lower and often variable temperatures. In the pharmaceutical world, a shift in melting point signals contamination, sometimes as little as half a degree. Manufacturing processes demand certain materials stand up to heat. Imagine airplane parts that soften in sunlight—that risk is cut by careful consideration of melting point.
Solubility: The Hidden Player
Without understanding solubility, nothing in this world would dissolve—from sugar in tea to medicines in the bloodstream. Solubility tells us how much of a substance fits in a liquid before the rest falls out. In my college years, I kept failing to dissolve salt in cold soda, but everything disappeared easily in hot water. That simple experiment made it clear: temperature, pressure, and solvent matter.
Farmers want fertilizers that dissolve just enough in rainwater, so plants get fed and nothing washes away before roots have a chance to use it. In medicine, poor solubility keeps promising drugs from working. Drug developers fight this every day, searching for new chemical combinations. A powder that won’t dissolve in your body might leave your medicine useless no matter how strong the dose. Poor water solubility blocks nutrients in food, too. Nutrition labels mean little if our bodies can’t access what’s inside.
Real-World Impact and Next Steps
Every industry touches these basics. Food scientists craft instant coffee that dissolves quickly and tastes right. Textile makers choose dyes that won’t wash out in the first rain. Water treatment plants rely on insoluble contaminants settling down, so clean water remains on top. Daily life would grind to a halt without these rules.
If science had all the answers, we wouldn’t see life-saving drugs stalled for years or plastics crumbling unexpectedly in heatwaves. The ongoing challenge is finding innovative ways to tweak these properties. Nanotechnology promises more control at a tiny scale—some nanoparticles dissolve more easily, improving drug delivery. Engineers design hybrid materials, combining the best of two worlds: resistance to heat, perfect solubility, or both.
Chemistry never stands still. New methods, like supercritical fluids and green solvents, are breaking old limits. Problems remain, especially around making discoveries safe for people and the environment. Industry and scientists have to keep adapting, measuring, and learning — just as that failed candle experiment taught me, the real world rarely behaves like the textbook.
Looking Inside the World of Niche Chemicals
People who wander into the world of research chemicals, especially unusual ionic liquids like 1-Allyl-3-Butylimidazolium Hexafluorophosphate, usually discover fast that not every question gets a neat, single answer. Even the seemingly simple ones—“what purity can I buy?” or “does it come in small bottles or by the drum?”—can turn into a rabbit hole. I’ve spent years talking to vendors, pouring over catalogs, and checking SDS sheets. I know that what’s written isn’t always what we get in the bottle.
Real Differences in Purity: Lab World vs. Market Realities
Most vendors selling this chemical list their purity grades. That’s one of the first things chemical suppliers highlight, especially for academic and industry buyers. Purities above 97% or even 99% pop up the most, since applications in electrochemistry, catalysis, or as solvents often demand it. Those numbers on a datasheet, though, don’t always mean the exact same thing. Sometimes it’s about the presence of water or leftover starting material. Other times, it’s less obvious contamination, such as trace metals barely mentioned unless you really pry. From my time in the lab, I learned that choosing between a 99% and a 97% sample sometimes feels less like picking “better or worse” and more like “how much uncertainty can I live with for this project?” If the purity isn’t clear, asking directly, or requesting a certificate of analysis clears things up—otherwise, buying blind burns both time and budget.
Packing Sizes: From Grams to Drums, With a Few Surprises
The other half of the story lives in the packaging. Small-scale researchers favor one-gram vials, ten-gram plastic bottles, or maybe a 100g glass flask if things get serious. It’s not because anybody wants to pay more per gram, but because budgets rarely leave room for waste. Suppliers know this and usually offer a “smallest available” that starts at a gram or five. For buyers with an industrial process or a pilot plant, 500g jars and even five-kilogram tubs become possible. My experience has shown that while some suppliers show off a huge menu—1g to 10kg—others quietly admit the bigger sizes only ship by special order.
Every packaging choice brings trade-offs. Small bottles limit risk and keep labs tidy, but they cost more for every milligram and fill up a waste container fast. Larger bottles save money but bring their own headache if the chemical sits around and degrades or absorbs moisture. Anyone who’s opened a sticky, half-used jar knows that feeling.
Choosing What’s Right—and Actually Getting It
This isn’t just a marketplace story. Researchers and buyers juggling between publication deadlines and financial limits find themselves comparing not just “what’s available” but “what’s practical.” Some suppliers give detailed COAs, while others handwave questions about impurities. It falls on buyers to stay skeptical and double-check certificates and batch numbers. I have found that it helps to ask three specific questions before hitting “order”: exact batch purity, date of packaging, and whether there’s an option for custom quantities. Sometimes these three things break a tie between vendors.
Improving the System: Transparency, Accountability, and Better Options
The answer lies in transparency. Suppliers who share detailed batch analysis, packaging dates, and shipping conditions gain trust. Education can help, too. Grad students placing their first chemical order don’t always get a primer in what a vague “>97%” or a half-worn bottle label really means for their experiment. A workshop or a guide on reading chemical specs gives early-career scientists more power.
Truth is, the world of specialty ionic liquids isn’t going to simplify overnight. For now, asking smart questions and refusing to settle for unclear answers stays the best way to steer through the thicket.