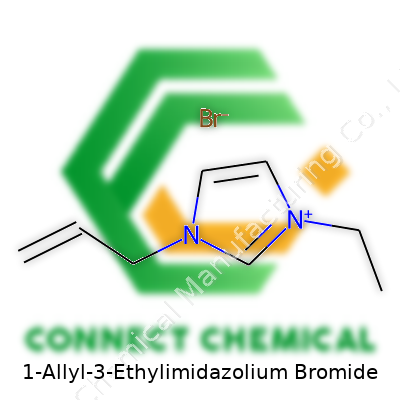
1-Allyl-3-Ethylimidazolium Bromide: A Comprehensive Commentary
Historical Development
Researchers have spent decades searching for safer, more adaptable solvents and materials for both industry and laboratory settings. The story of ionic liquids began in the mid-20th century, with breakthroughs in the late 1990s when substances like 1-Allyl-3-Ethylimidazolium Bromide (often abbreviated as [AEIm]Br) took center stage. Chemists realized that tailoring the cation and anion composition in imidazolium-based ionic liquids offered a shot at fine-tuning properties for specific uses. Once a novelty among small academic groups, [AEIm]Br steadily worked its way into a range of scientific journals, conference proceedings, and patents, as practical experiments demonstrated its promise in catalysis, extractions, and material synthesis. This rise reflected a broader trend in chemistry: move away from volatile organics and carve out new frontiers in green chemistry.
Product Overview
1-Allyl-3-Ethylimidazolium Bromide belongs to a family of salts that stay liquid near room temperature. The compound typically appears as a colorless to pale yellow viscous liquid, combining an allyl group, an ethyl group, and an imidazole ring, paired with a bromide counterion. The structure gives it a unique set of properties, valued by those intrigued by sustainable and functional materials. [AEIm]Br often enters labs in carefully sealed bottles to protect it from moisture, since the ionic nature leads it to draw in water, sometimes swelling or clumping over time.
Physical and Chemical Properties
This compound preserves a liquid state well below the boiling points of traditional salts. Its melting point generally falls below 50°C, due in large part to the asymmetry in the organic cation. [AEIm]Br stands out with high polarity, low vapor pressure, and substantial ionic conductivity, resisting evaporation even under reduced pressure. The bromide anion makes the substance prone to halide exchange reactions and offers moderate hydrophilicity. The viscosity can pose a challenge during certain mixing processes, but with mild heating it flows much more easily. The density, slightly heavier than water, changes little with minor temperature variation. Chemists find the decomposition point important, as [AEIm]Br usually begins to break down at temperatures above 180°C, forming volatile components or char in severe cases.
Technical Specifications and Labeling
Manufacturers of [AEIm]Br usually provide detailed data sheets with physical constants such as purity (above 97% for most applications), water content (below 1% preferred), appearance, molecular weight (approximately 249.1 g/mol), refractive index, and thermal stability limits. Labels warn against exposure to air and moisture, as the compound may clump or react over time. Standard packaging often includes lot numbers and storage instructions, encouraging storage below 30°C in an airtight environment, away from both oxidizers and acids. Details about possible halogen impurities or transition metal residues appear in technical bulletins, helping chemists troubleshoot reactions or processes down the line.
Preparation Method
Synthesis usually involves quaternization of 1-ethylimidazole with allyl bromide. Researchers combine the components in an appropriate solvent (often acetonitrile or dichloromethane) and control the reaction temperature near room conditions for several hours. After the quaternization, the solvent gets evaporated under reduced pressure, leaving behind the crude product. Purification might involve multiple solvent washes, sometimes even column chromatography, to remove side products or reactant leftovers. Laboratories performing scale-ups use inert atmospheres and dry conditions to keep water out, minimizing hydrolysis or unwanted side reactions. Waste streams get managed carefully, especially because bromide waste poses environmental hazards. Some commercial providers use advanced ion-exchange or recrystallization processes to obtain exceptionally pure [AEIm]Br for sensitive applications.
Chemical Reactions and Modifications
[AEIm]Br takes part in various chemical reactions due to its nucleophilic and electrophilic sites. The allyl group makes the cation susceptible to addition reactions, especially with electrophiles. The bromide anion readily undergoes metathesis—allowing replacement with other anions to create a wide variety of ionic liquids. Some researchers use [AEIm]Br as a template or co-catalyst in organic synthesis, where the ionic nature can stabilize transition states and increase reaction efficiency. Surfaces functionalized with [AEIm]Br moieties attract attention for catalysis, where the imidazolium core offers both thermal and chemical stability. On an industrial scale, modified versions see use in polymerizations, forming gels or membranes with ionic conductive properties.
Synonyms and Product Names
1-Allyl-3-Ethylimidazolium Bromide carries several labels across catalogs and research papers. Common abbreviations like [AEIm]Br or AEMImBr appear in literature. Synonymous chemical names include 1-ethyl-3-allylimidazolium bromide and ethyl(allyl)imidazolium bromide. Some suppliers list the product with these alternate names, so one bottle may bear a slightly different label than another, even for essentially the same product. Matching the molecular formula (C8H13BrN2) or CAS number (74411-65-1) ensures researchers work with the intended substance.
Safety and Operational Standards
Handling [AEIm]Br calls for good laboratory practices. Contact with skin, eyes, or inhalation can provoke irritation, so gloves, goggles, and sometimes fume hoods become part of the daily routine. Although not as volatile as standard solvents, spills can lead to slippery surfaces and environmental concerns, given the bromide ion’s reactivity. Disposal often calls for neutralization followed by collection through licensed waste channels, in compliance with local hazardous substances regulations. Laboratories expecting larger volumes set up spill response kits nearby. Safety data sheets also mention storage stability, noting how light and atmospheric exposure can degrade product quality over time.
Application Area
[AEIm]Br earns its respect in a wide range of industries and laboratories. In green chemistry, chemists recognize it for minimizing the use of toxic, volatile organics. Researchers exploring cellulose dissolution find its ionic properties invaluable, providing a less hazardous route for biomass conversion. In organic synthesis, [AEIm]Br helps stabilize interactions in transition-metal catalysis and supports formation of complex molecules that struggle in other solvents. Electrochemistry benefits from the ionic conductivity and thermal range, supporting advanced sensors and batteries. In analytical chemistry, [AEIm]Br contributes to extraction efficiency for hard-to-separate natural products or pharmaceuticals. Some industrial players experiment with the compound as a building block for custom liquid electrolytes, finding benefits in both efficiency and process safety.
Research and Development
Laboratories constantly tinker with the structure and performance of [AEIm]Br. Ongoing projects seek to pair the cation with new anions, each one targeting different physical or chemical profiles. Publications in journals like 'Green Chemistry' and 'Journal of Physical Chemistry B' highlight not only synthesis improvements but core applications in catalysis, separation science, and even nanomaterial growth. Graduate students and chemical engineers run head-to-head experiments with [AEIm]Br against traditional solvents, measuring product yields, purity, and environmental impacts. Funding agencies have paid close attention to the promise in battery and fuel cell technology, supporting pilot projects in collaboration with both chemical manufacturers and tech startups.
Toxicity Research
Health and environmental safety guide much of the current research. Published toxicity studies reveal moderate ecological effects, primarily linked to the bromide ion and the persistence of the imidazolium core in soil or water. Acute toxicity in humans remains low for small laboratory contacts, though chronic exposure data remain limited. Bioaccumulation studies point to slow degradation, which could pose long-term environmental stress if disposal practices slip. Regulatory agencies suggest regular reviews of workplace exposure data, and researchers recommend improved containment and decontamination protocols. Several recent studies test modified analogs to tackle these concerns, using greener anions or biodegradable cation variants, although effective replacements need to match [AEIm]Br’s performance in specific chemical pathways.
Future Prospects
Application trends point toward greater uptake of [AEIm]Br in sustainable chemical technologies, battery science, and green synthesis. As industries tighten the reins on safety and environmental performance, compounds that offer low volatility and high chemical adaptability find a ready audience. Ongoing research will likely deliver new blends and formulations, addressing toxicity concerns while expanding the list of compatible chemical processes. As knowledge deepens, chemists may well uncover routes for more cost-effective, large-scale synthesis paired with safe end-of-life management. Those investing in R&D keep tabs on regulation, evolving safety practices, and the emerging global market for specialty ionic liquids. Where the next breakthrough takes place could hinge not only on chemistry but also on creative thinking about resource recovery, lifecycle analysis, and partnerships across disciplines.
Getting to Know the Compound
Digging into the world of ionic liquids always takes you to some strange corners of chemistry, but few stand out like 1-Allyl-3-Ethylimidazolium Bromide. This compound belongs to the imidazolium family. The name sounds a bit intimidating—probably the sort of thing most folks have never heard of outside of academic journals. But chemists know this salt as the kind that stays liquid under pretty tame conditions, without needing much in the way of heat.
Dissolving the Problem: Its Power in Cellulose Processing
One place where this compound pops up on a regular basis: cellulose dissolution. For as long as I’ve watched the green chemistry crowd, the challenge has circled back to breaking down plant fibers. Cellulose holds up tree trunks and gives cotton its strength, yet dissolving it has eaten up massive resources for years. Between the tough molecular structure and the general stubbornness of cellulose, most solvents just bounce off.
1-Allyl-3-Ethylimidazolium Bromide changes the game. The compound can tear cellulose apart where water or standard organic solvents fail. It keeps the process stable, with little volatility or vapor. Biofuel researchers and folks hoping for sustainable plastics count on it to dissolve plant material without complex setups. The science backs this up—one 2020 study from Materials Today Chemistry detailed its effectiveness at breaking hydrogen bonds in natural fiber.
Why This Matters
Getting cellulose into a usable form can make or break progress in renewable materials and biofuels. In my own lab experience, nothing saps motivation like endless mixing and filtering with traditional acids or copper solutions, all ending in endless cleaning. Using this compound made it possible to get high purity output and less headache with waste. It isn’t just academic; businesses use the dissolved cellulose to spin new fibers for textiles, make cellulose films, or ferment sugar-rich hydrolysate into ethanol.
Where the Road Gets Bumpy
Every new tool brings its baggage, and no compound gets off scot-free. The high cost of making these ionic liquids keeps the scale a bit low for massive industrial operations. There’s also the challenge of recycling these solvents. They don’t just decompose in the open air, and careless use can throw up environmental concerns. Add to that the need for careful water removal—wet solvents lose a lot of their punch.
Lab scale methods work, but shifting to actual factory floors calls for more budget and clever design. I've seen some teams recover and reuse the liquid several times, filtering out the gunk each round. Some groups explore hybrid solvent systems to stretch out the effective life of the compound.
Finding a Better Way Forward
Progress tends to come from the most persistent tinkerers. Efforts ramp up around lowering the price of production using cheaper feedstocks. Greener methods for recovery and regeneration show up in new papers every few months, giving hope to everyone hustling for more sustainable cellulose use. I’ve watched groups experiment with closed-loop systems and improved purification techniques, promising to shrink both waste and costs.
Watching this field over time makes it clear: 1-Allyl-3-Ethylimidazolium Bromide didn’t land by accident as a workhorse in cellulose breakdown. The work isn’t done, but today’s materials science rests on the promise these new solvents bring to natural polymers—far beyond what anyone could get with traditional chemistry.
Breaking Down the Name
Chemistry names can sound intimidating, but at their core, they’re detail-packed instructions that describe exactly what’s in a compound. With 1-allyl-3-ethylimidazolium bromide, each part of the name offers clues: the "imidazolium" backbone means two nitrogen atoms sit in a five-membered ring with three carbons. One nitrogen sports an allyl group (a chain of three carbons with a double bond), while the other has an ethyl group (a two-carbon chain). The "bromide" part tells us a bromine anion balances the positive charge from the ring.
Chemical Formula Details
Stringing these bits together, scientists write its formula as C8H13N2Br. Anyone working with this substance can check this formula for ordering, safety data sheets, or research. It’s easy for a small change—a missing hydrogen, an extra methyl group—to throw off results, so accuracy matters.
Molecular Weight: Why the Numbers Matter
I’ve watched lab teams fuss over grams and milligrams. The molecular weight, or molar mass, lands at 233.11 g/mol. This number comes from adding the atomic weights of every atom: carbon (12.01), hydrogen (1.008), nitrogen (14.01), and bromine (79.90). No shortcuts here—correct measurements let teams make right concentrations, avoid costly waste, and run safer experiments.
Precise numbers add up to much more than just tidy records. Say, in pharmaceutical studies, the difference between a fraction of a gram can play out in dosing, toxicity, and how a compound interacts with the body. Slip-ups in molecular mass calculations can lead researchers to the wrong conclusions or risk safety—there’s been enough scientific retraction tied to poor diligence in simple details like these.
Why This Compound Pops Up in Modern Chemistry
I remember years ago, ionic liquids sounded almost exotic. Now they’re everywhere, and 1-allyl-3-ethylimidazolium bromide is a good example of their rise. Its low volatility and robust thermal stability make it valuable for green chemistry, as it doesn’t evaporate into the air like many traditional solvents. Labs see less waste, and technicians: fewer headaches caused by fumes. Its formula and weight land it among customizable solvents for everything from catalysis to advanced materials research.
This compound rarely appears alone. Partners in research use it to dissolve cellulose, enable electrochemical processes, or boost the efficiency of certain extractions. In my own work, swapping out more hazardous chemicals for substances like this has made the benchwork less stressful and the cleanup simpler. Knowing its makeup makes risk assessments and safety reviews more transparent.
Steps Toward Safer, Smarter Chemical Use
The right data sets a strong foundation for lab safety and progress. With dangerous mix-ups still cropping up in industry news, more chemists insist on double-checking chemicals before any experiment begins. Needed improvements include open safety databases, more thorough safety training, and wider sharing of peer-reviewed protocols. Accidents decrease when everyone can trace each substance back to its precise formula and weight.
Having solid data on 1-allyl-3-ethylimidazolium bromide means students, researchers, and industry folks can all stay a step ahead. That kind of transparency not only supports discoveries but protects people every day.
Why Thoughtful Storage Matters
1-Allyl-3-Ethylimidazolium Bromide, a specialty ionic liquid, pops up in laboratories and some industrial setups. Like a lot of ionic liquids, it can be stable in a range of conditions, but that doesn’t mean tossing it on a random shelf and hoping for the best. My personal experience with storing sensitive chemicals has taught me that skipping careful storage ends up as a headache down the line, whether that’s leaks, ruined reagents, or worse, safety incidents. The chemical can absorb water from the air, and its reactivity can change if handled carelessly, so it's worth paying attention to how you stash it away.
The Trouble with Humidity
Left open to air, this compound starts soaking up moisture. That water can build up and mess with experiments, especially where consistency and purity matter, such as catalysis or organic synthesis. I remember working on a project where trace water from poor storage completely skewed results. With a chemical like this, it's best to use airtight containers made out of glass or high-quality plastics. Seal them tight after every use, and pick containers that can handle repeated opening and closing.
Keep it Cool, Keep it Dark
Direct sun and high heat often ruin good reagents. Sunlight can speed up decomposition, and temperature swings cause condensation that creeps into bottles. In my years in the lab, the best results always came from storing chemical stocks in a dedicated cabinet away from light, often kept at room temperature. A dedicated fridge (labeled for chemicals only, not for food) works if climate control in the room is spotty. No one wants to stumble across a container that’s weeping or full of lumps—steady, moderate conditions win every time.
Avoid Mixing Up Labels and Containers
Mislabeling or reusing old bottles leads to real risks. I’ve seen colleagues frustrated after grabbing a container, only to realize later the contents weren’t what they expected due to a missing or faded label. Start by writing the chemical name, concentration if it's mixed, the date it was opened and who handled it last right on the bottle. Keep original manufacturer labels whenever possible. If a transfer into a new container proves necessary, select high-quality bottles and transfer all relevant labeling information, never taking shortcuts.
Protect Yourself and Others
Proper lab safety means more than eye goggles and gloves. Chemical spills or degraded substances put people at risk, especially if storage gets sloppy. Setting aside a dedicated spot for ionic liquids and their relatives lowers the chances of someone grabbing the wrong chemical or using it where it doesn’t belong. Place spill kits, absorbent pads, and eye-wash bottles nearby. Sharing safety reminders with coworkers and leaving helpful notes or reminders around storage sites raises awareness for everyone involved.
Disposal Practices Prevent Problems
Old or contaminated 1-Allyl-3-Ethylimidazolium Bromide doesn’t belong in regular trash cans or down the drain. I once watched a cleanup derail an entire afternoon because someone ignored disposal protocols. Work closely with EHS teams on campus or in the workplace to arrange hazardous waste collection. If in doubt, it pays to call in professionals who know the regulations for safe chemical disposal. Responsible storage always pairs with thoughtful disposal practices—protecting people and the environment from unnecessary exposure.
Understanding the Role of 1-Allyl-3-Ethylimidazolium Bromide
1-Allyl-3-ethylimidazolium bromide comes up a lot in labs and industry circles. People refer to it as an “ionic liquid.” It dissolves a wide range of stuff, often serves as a solvent in chemical syntheses, and researchers like to push it for cleaner or “greener” projects.
Safety Concerns: What Actually Happens When People Handle It
Every chemical carries some risk, even those calling themselves eco-friendly. The best way to look at 1-allyl-3-ethylimidazolium bromide starts with its basic properties. It isn’t volatile and doesn’t smell strong. You’re not likely to breathe it in unless someone mists it around, which almost no one does in a responsible lab. The main route into the body comes from skin contact or, by mistake, swallowing it.
I’ve worked with ionic liquids in lab settings, and nobody just splashes these on their hands. Standard guidance calls for gloves and goggles, mainly because such compounds can irritate the skin or eyes. There’s not a big pile of evidence showing this particular ionic liquid to be highly toxic in small amounts, but the imidazolium group as a class sometimes shows moderate toxicity—cell cultures and aquatic species react when the concentration spikes.
Environmental and Health Impact: Known Facts
Most of what we know about environmental harm comes from related chemicals. Ionic liquids break down slower than table salt or acetone; they tend to stick around in water or soil. Some data suggest they harm aquatic life, disrupting cell membranes after repeated or heavy exposure.
Very few people would dump buckets of this into drains or rivers, but smaller spills still raise questions. Regulatory guidance in Europe (REACH database) and in research papers agrees on one point: treat even the “greenest” ionic liquids with the same caution as any new lab chemical until more data shows otherwise.
Reducing Risks: Smarter Handling, Better Practices
No shortcut here — working safely makes all the difference. Any lab should require gloves, good ventilation, and splash-proof eye protection. Clear labeling and good housekeeping keep accidents down. My time in industry taught me never to trust a “safe” label alone. Reading the safety data sheet every single time matters more than chemical claims or sales promises.
Disposal also needs real attention — not every chemical waste stream treats ionic liquids the same way as common solvents or salts. Companies and universities need to check if their treatment facilities can handle these compounds without sending them through regular waste lines.
Building a Knowledge Base: What’s Missing and What to Ask
As a class, ionic liquids still show a lot of unknowns. Researchers have only begun to scratch the surface about their breakdown products or low-dose, long-term impact. People working in labs, teaching, or supervising students should push for real-time toxicity checks, especially where those liquids might end up outside the building.
The smart move has always been to err on the cautious side. Anyone working with new solvents, no matter how “green” the pitch, should match their habits to those used for classics like acetone or benzene. No short supply of gloves ruined or chemical hoods running overtime ever cost more than the price of one bad exposure. That’s one lesson that hasn’t changed, no matter what name the latest chemical wears.
Understanding Purity Claims
Buying specialty chemicals like 1-Allyl-3-Ethylimidazolium Bromide brings up one big question: how pure is it, really? Posted purity levels often sit between 95% and 99%. Not every supplier means the same thing by those numbers, though. Some focus on main component content; some factor in water and trace salts. Cost and reliability swing with the details. Many researchers rely on certificates of analysis, but not every vendor provides them or gives full test methods. Labs stuck with an “off-the-shelf” batch can't guarantee consistent results if tiny contaminants sneak in.
Impact on Research and Industry Use
Purity isn't just a number for the sake of bragging rights. Applications in catalysis, electrochemistry, or green solvent development can suffer from trace organics or halide by-products. One time, a chemistry colleague spent weeks puzzled over sloppy reaction yields, only to find that a minor impurity in their ionic liquid poisoned a catalyst. Real money gets wasted on reproducibility problems. Some groups working with ionic liquids report that going up just a couple percent in purity has changed electrochemical windows and NMR spectra noticeably. Recrystallizing or re-purifying costs precious project time.
Market Realities and Costs
Suppliers across regions—China, Europe, North America—tend to advertise purities over 95%. Above 99%, expect to pay significantly more and sometimes wait for custom synthesis. For industrial-scale projects, vendors sometimes cut corners to win bids, so any purport of “high purity” without third-party verification should raise an eyebrow. On top of that, storage and shelf life can affect long-term purity, since hygroscopic ionic liquids pull in moisture and sometimes react with air.
What Lab Users Can Do
In practice, anyone running sensitive experiments builds in a layer of skepticism with new batches. One approach involves testing a small sample—NMR, Karl Fischer, and conductivity checks figure into many routines. Asking suppliers for batch-specific certifications and impurity breakdowns helps, even if it feels like a hassle. Direct conversations with technical support usually shake out the differences between “assay” and “purity.” Some bulk buyers negotiate for regular, independent quality checks at delivery.
I learned early on to set aside funding for basic quality control tests and treat “analytical grade” as a claim, not a promise. Adopting this habit avoided false starts that drained both budget and morale. For those in teaching labs or preliminary studies, going with 95% purity may work out, but nothing replaces hands-on validation for advanced applications.
Push for Better Transparency
Global trends nudge manufacturers toward better reporting and product traceability. Journals and funding bodies show more interest in granular methodology descriptions and batch documentation. For anyone planning a project around ionic liquids, build in time for purity checks, expect some variance, and factor in the hidden costs surrounding this “simple” specification. Chemistry always rewards those who treat raw material specs as the start of a conversation.