An In-Depth Commentary on 1-Allyl-3-Ethylimidazolium Tetrafluoroborate
Historical Development
The roots of ionic liquids reach back to the middle of the last century, but 1-allyl-3-ethylimidazolium tetrafluoroborate got its real shot in the late 1990s, when chemists started pushing beyond chloroaluminate salts. By the early 2000s, researchers had tuned in to the possibilities offered by imidazolium-based ionic liquids like this one, especially for their low volatility and high thermal stability. The drive for safer, greener storage, and process-friendly reaction media picked up speed as academic and industrial labs started replacing volatile organics with salts such as this. The chemical industry’s longstanding reliance on crude and corrosive solvents led chemists to chase after more user-friendly alternatives, and ionic liquids like this one became a key figure in bench and pilot plant work. The development didn’t happen in a vacuum—think back to skyrocketing concerns over emissions, the push for green chemistry, and strong interest in recyclable solvents. Those trends shaped the direction for research and development, and this compound soon found advocates across synthetic organic chemistry, catalysis, battery research, and materials science.
Product Overview
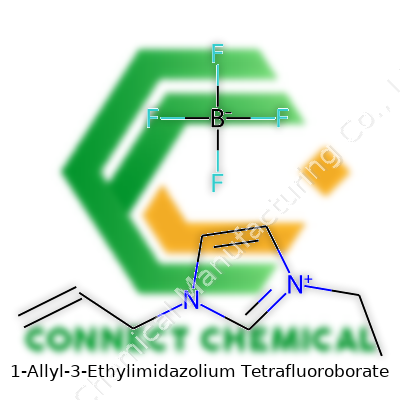
1-Allyl-3-ethylimidazolium tetrafluoroborate attracts attention for its versatility and distinctive liquid state at room temperature. This compound consists of an imidazolium cation substituted at the nitrogen atoms with an allyl group and an ethyl group, paired with a tetrafluoroborate anion. Known for its far lower vapor pressure compared to traditional organic solvents, its oily consistency, and high polarity, it has taken up space in settings from laboratories to early-stage manufacturing pilot projects. Its chemical makeup gives it broad compatibility with a range of polar and nonpolar substrates, opening doors where other solvents fall short, and its unique electrical properties bring added value to energy storage and transport applications.
Physical & Chemical Properties
You won’t mistake this clear to light yellow liquid for water: it carries notable density, typically logging in around 1.1 to 1.2 g/cm³. It remains fluid in a broad temperature window, holding together at temperatures well above 100°C without decomposing. Unlike many volatile solvents, it won’t fill a room with fumes; its vapor pressure stays very low under ambient conditions. Heat it, and it still holds together without giving off much odor or easily crossing the line into volatility. Water solubility tends to fall in the moderate range, which lets it blend into aqueous media or get separated after reactions, depending on the context. Chemically, the tetrafluoroborate anion resists hydrolysis, which means fewer headaches with product breakdown or unwanted reactivity in wet environments.
Technical Specifications & Labeling
A typical bottle will show a purity at or above 98%, commonly offered in glass or specialized high-density polyethylene bottles to avoid leaching or contamination. Labels for laboratory or pilot use need to cite molecular weight, batch number, storage requirements, hazard symbols, and supplier contact information, along with date of manufacture. Specific gravity, conductivity, residual halide content, and moisture content also show up on more detailed technical data sheets, since each of these properties can influence outcomes when you scale up a process or dig deep into quality control. Compliance with REACH, GHS, and common practices for safe labeling in chemical regulatory regimes ensures consistent handling from lab bench to scale-up bay.
Preparation Method
Lab-scale synthesis starts by quaternizing 1-ethylimidazole with allyl chloride to form the core cation. This intermediate salt then reacts with sodium tetrafluoroborate in a metathesis step, typically in aqueous media, exchanging the halide for the BF4− anion. After cooling and phase separation, purification steps take center stage, including water washing, rotary evaporation, and vacuum drying to chase away any halide impurities and excess water. Monitoring pH, conductivity, and chloride content provides on-the-fly checks for purity before bottling. Even though large-scale production uses much of the same chemistry, engineers rely on continuous-flow reactors and automated purification to cut down on labor and improve reproducibility.
Chemical Reactions & Modifications
The imidazolium ring’s allyl group opens doors to further chemistry, especially in processes aiming for polymer attachment or grafting onto larger molecules. The compound holds up well under acidic and basic conditions but doesn’t take kindly to strong nucleophiles or extreme heat, which can crack the BF4− anion and send free fluoride flying. In electrosynthesis, it plays a critical role as both medium and sometimes catalyst, keeping reactive intermediates solubilized and supporting high ionic conductivity. Researchers have grafted the core structure onto silica supports, attached it to macromolecules, or swapped in different anions for tuning reaction outcomes or solubility in multistep processes.
Synonyms & Product Names
You’ll spot this compound under several names: AEMIM-BF4, 1-allyl-3-ethylimidazolium tetrafluoroborate, and its CAS number, 311383-20-7, all pin it down. Suppliers stick with the IUPAC or common abbreviations, but in published literature, expect to see a flurry of shorthand labels in experimental sections—AEMIM-BF4 or just “imidazolium IL (BF4),” depending on the journal and chemistry subfield. Brand names stay less prominent for research-grade salts; most researchers rely on catalog names when ordering samples for their projects.
Safety & Operational Standards
Handling any ionic liquid demands respect, but this one requires particular caution with personal protective equipment and good ventilation. Direct skin contact leaves oily residues; eye splashes build up irritation. Safety data sheets flag it as an irritant and call for goggles, gloves, and in cases of scale-up or prolonged work, use of chemical fume hoods. Disposal runs through collection as a halogenated organic, avoiding drains or evaporation to the air. Fire risk stays low under normal storage conditions—thanks to its high flash point and non-flammable nature—but mixing with oxidizers or heating to extremes can produce harmful decomposition products such as hydrogen fluoride. Chemical storage norms call for airtight containers away from acids, bases, and oxidizers with clear hazard labeling. Facility-level best practices include spill trays, chemical event logging, and periodic review of procedure updates as newer information comes out.
Application Area
This ionic liquid has carved out places in catalysis, extraction, electrochemistry, and as a tool for material scientists chasing new nanocomposites or electrode coatings. In my own work, I’ve relied on it to support the electroplating of metals where hydrogen bubbling would usually ruin surface finishes or introduce defects. It’s earned a reputation for supporting complex organic coupling reactions that need a strongly polar but non-volatile medium. Battery researchers value its wide electrochemical window, tapping it for non-aqueous electrolytes in supercapacitors and advanced lithium systems. Industrial teams have explored it for separating rare earths or precious metals through extractive processes where the old solvents fall short by causing emulsions or high toxicity. In polymer chemistry, it doubles as a solvating agent and a stabilizer for living radical polymerizations, helping drive clean, controlled chain growth.
Research & Development
Recent research keeps pushing into eco-friendly processes and chemical recyclability, priorities that only gain steam as green chemistry goals hit mainstream labs. Peer-reviewed studies trace benefits in biomass conversion, including cellulose dissolution, and in pharma process intensification, where traditional solvents cause more waste or lower purity. Teams tackling supported ionic liquid phase (SILP) catalysts have spotlighted this ionic liquid in the push for recyclable fixed-bed reactors. My own time in a catalysis lab saw its use for extracting catalysts that otherwise stuck to product phases or fouled valuable membranous filters. The next crop of papers eyes broader anion swaps and ring-modified derivatives designed to further tune solubility, redox properties, and toxicity profiles for specialized needs.
Toxicity Research
Toxicological studies reveal that while the imidazolium backbone shows reduced volatility and less bioaccumulation compared to many chlorinated solvents, it still brings moderate aquatic toxicity. Chronic exposure studies point to stress effects in freshwater invertebrates at mid-to-high ppm, mainly driven by the BF4− anion’s fluoride content and the cation’s persistence in water and soil matrices. Research has not shown strong oral toxicity, but skin or eye irritation risk remains, especially in chronic or large-scale occupational settings. My contact with regulatory teams over the last decade boiled down to a single point: always err on the side of containment, waste treatment, and incident reporting when working at volumes above bench scale. More recent findings highlight breakdown products as risks if incinerated improperly, so incineration with fluoride capture or specialized chemical waste treatment has become standard for cleanup efforts.
Future Prospects
Interest in this ionic liquid looks set to keep growing, especially as industries confront mounting regulations on VOC emissions and hazardous solvent disposal. The trend leans toward blending ionic liquids with other advanced materials—ceramic electrolytes, polymer matrices, or molecular sieves—to boost selectivity, durability, or process safety. Energy storage and conversion projects call for steady and reliable electrolytes, so work on tailoring these salts to work hand-in-glove with specific battery chemistries or catalysis platforms continues to gain traction. With more advancements in biodegradable or structurally tunable anions, versions of the classic imidazolium architecture promise even lower toxicity and better lifecycle profiles. Real progress will depend on continued collaboration between academia, manufacturing, and regulatory bodies to balance utility and safety. Teams that keep up with the emerging standards and adopt greener synthesis routes will set the pace as regulatory rules get more demanding and industrial applications expand into energy, environment, and specialty chemical spaces.
Not Your Everyday Household Name, but a Heavy Lifter in Labs
Most people don’t talk about 1-allyl-3-ethylimidazolium tetrafluoroborate at the dinner table. Chemists sure do. This ionic liquid works as a solvent where water or regular organics just can’t cut it. Scientists put it to work dissolving tough compounds in processes that feed directly into new batteries, advanced drug delivery, and clean energy. It isn’t thick like honey, or watery like, well, water. It brings a slippery, non-volatile character to the bench—safer to use and less likely to evaporate out of the flask mid-reaction.
Why Go for an Ionic Liquid?
Organic solvents, like acetone or toluene, have a habit of catching fire or harming your lungs. This one doesn’t. Its unique recipe makes experiments less risky in crowded university labs and massive industrial reactors alike. Even regulators see the upside: fewer toxic fumes, less waste pouring down drains, and a lower carbon footprint.
Batteries, Green Chemistry, and Next-Gen Manufacturing
Energy storage shapes up as one of the big battlegrounds for technology. Companies working on new supercapacitors or lithium-air battery designs often need a stable ion transporter—a liquid that shuttles charge from point A to B without eating away electrodes or gumming up the works. This salt-based solvent sticks around under voltage and heat, letting these devices crank out more cycles before losing their punch.
I saw researchers at an energy startup soak test cells in this compound, then compare the results against older, more volatile chemicals. Their best-performing batteries held up for hundreds of cycles. That’s a huge leap for grid storage, especially when the world keeps demanding more reliable renewables.
Unlocking Biomass and Cleaner Synthesis
Not every molecule plays nice with water and alcohols. When chemists try to break down wood or transform agricultural waste into biofuels, they need a “magic sauce” to take apart stubborn lignin, cellulose, or other plant guts. 1-allyl-3-ethylimidazolium tetrafluoroborate helps rip those bonds, freeing up sugars for fermentation or transformation into plastics. Better still, factories can use this solvent over and over, slashing chemical waste—a big deal for greener production lines.
Pharmaceutical chemists leverage this same solvent to drive reactions that demand careful temperature control and strong dissolution power. That unlocks access to rare intermediates, some of which evolve into advanced cancer drugs or more effective antibiotics. The pharmaceutical world doesn’t gamble with purity or safety. This solvent lets researchers meet those standards without reaching for hazardous organics.
Steering the Conversation Toward Safer Industry
Chemistry’s dark past brims with solvents that poisoned water, sickened workers, and sparked warehouse fires. These ionic liquids aren’t perfect—tetrafluoroborate has its own waste handling protocols—but the shift marks a step toward smarter, more responsible manufacturing. Getting there means honest conversations about chemical sourcing, lifecycle analysis, and teaching new generations of chemists how to balance results with stewardship.
Future Possibilities Laid Bare
This compound won’t fix every problem with one easy swap. What it offers is a new tool for industries trying to boost performance while doing less harm. Governments can keep supporting research on safer solvents, industry leaders can invest in closed-loop recycling, and schools ought to teach chemists why green choices pay off—not just for profits, but for people breathing the air and drinking the water downstream.
The Realities of Lab Work
Anyone who works around chemicals knows the drill: safety glasses, gloves, a splash-proof coat, and a working fume hood. The challenge comes with substances that look harmless on paper and in the bottle, but turn out to be wolf in sheep’s clothing. 1-Allyl-3-ethylimidazolium tetrafluoroborate – often shorthanded as an ionic liquid – falls into that camp. It’s transparent, nearly odorless, and not prone to igniting or evaporating, so it lulls you into thinking it’s nearly as safe as water. But there’s no shortcut to safety, and this chemical demands caution.
Where Ionic Liquids Get Tricky
Ionic liquids have a reputation for being “green solvents.” People like to point out how they don’t release a cloud of vapor, and how you can use them again and again in a reaction setup. That sounds tempting for improving sustainability in the lab. But experience teaches that labeling a chemical "green" often just means the risks show up in a different way. In this case, the tetrafluoroborate anion breaks down under the heat of a reaction or in the presence of acids and bases. That releases hydrogen fluoride (HF), a gas with a nasty way of sneaking past gloves and skin to cause painful burns and systemic toxicity.
It only takes a few stories from colleagues – the painful tingling, chalky white burns that don’t show up until hours after exposure – for safety concerns to start feeling real. Literature and Material Safety Data Sheets (MSDS) warn about this, easily confirming these stories aren’t just lab legends. A compound that can turn into HF deserves respect, no matter how stable or “environmentally-friendly” it seems at first glance.
Staying Ahead of the Risks
Years of running reactions with ionic liquids have shown that the main danger comes from getting too comfortable. Short sleeves, old gloves, or skipping the face shield open the door to accidents. Mistakes happen, pipettes break, droplets fly, and suddenly you’re cleaning up not just a spill, but a potential source of serious harm. The solution isn’t to swear off useful solvents, but to double down on basic safety: nitrile gloves in good condition, splash-proof goggles, proper waste containers, and a fume hood with real airflow.
Most university and industry labs have started upgrading safety training around HF-releasing compounds, but there’s still ground to cover. Teaching just the MSDS sheets is not enough. New lab workers do best when they see senior scientists model correct habits, explaining their process. No chemical’s reputation – good or bad – replaces seeing procedures in action. Safer handling comes from practical knowledge and mentoring, not just paperwork and online modules.
How to Handle and Dispose
Anyone disposing of ionic liquids with tetrafluoroborate must remember that standard drain disposal does not apply. Waste should go in a sealed, labeled container, marked as potentially HF-generating, and handed to a licensed disposal provider. I’ve seen labs forget this step, and pipes in the back rooms corrode months later. If your lab or facility ever runs out of proper containers, don't attempt improvisation or store in glass, as fluoride ions eat through glassware over time.
At the end of the day, 1-allyl-3-ethylimidazolium tetrafluoroborate offers lab techs and researchers some useful properties, but every upside comes with a demand for respect. Never take the clear, gentle liquid at face value. The greatest tool is steady, real-world respect for what a chemical can do – and passing that down, one generation of scientists to the next.
Crucial Details Get Overlooked
Often, people look past storage and handling when they deal with a product. Safe storage and handling don’t just keep things organized. They keep people safe, guard a company’s investment, and protect the product’s quality. I have seen cases where a small mistake—like keeping a temperature-sensitive product too close to a window—cost a warehouse thousands. Experience taught me that ignoring these details brings trouble down the line.
Temperature and Humidity Aren't Optional Considerations
Plenty of products, especially anything pharmaceutical, food-based, or chemical, demand a specific temperature range. Food spoils, chemicals decay, and pharmaceuticals lose strength if storage rooms get too hot or too cold. In my time managing a distribution center, I learned that keeping the thermometer in check matters just as much as tracking inventory levels. Regulatory agencies like the FDA and OSHA set strict rules for storage spaces, tracking conditions, and even staff training because mistakes can harm health or the bottom line. Humidity sneaks up just as quickly—extra moisture ruins paperwork, warps packaging, and encourages mold growth. Desiccant packs and dehumidifiers help, but staff also need to check storage logs and air vents on a daily basis.
Ventilation and Containment
Certain powders, chemicals, or fresh goods react badly to stale air. Fumes, vapors, or dust can build up fast, sometimes with dangerous results. I once watched an HVAC problem force a factory shutdown after off-gassing triggered air-quality alarms. Good systems remove hazardous fumes, protect employees, and keep the air clean around sensitive inventory. Not every workspace can afford a top-of-the-line ventilation upgrade, but basic air circulation paired with well-marked hazard areas reduces risk.
Pest Control Saves Money and Health
Rodents and insects don’t care about warehouse rules. One season, a missed crack in our storeroom door brought in ants that chewed packaging and contaminated stock. We lost good money and good client trust. Regular inspections, sealed doors, and simple traps make a difference. Employees who spot droppings or damage can take quick action, stopping an infestation before it grows. A walk-through checklist, signed each shift, boosted our awareness and caught most problems before they exploded.
Handling That Puts Safety Ahead of Speed
Speed isn’t everything. Rushed handling increases product breakage, injuries, and legal hassles. Lifting gear, gloves, and clear walkways help everyone work safer. Whenever new staff join, refresher training in equipment and spill response stops accidents before they happen. More than one emergency room visit stems from lifting wrong or ignoring the signs posted by the loading dock. I have witnessed how a few minutes invested in careful handling pays off with fewer claims and less waste.
Simple Tracking Prevents Complex Problems
Paper logs, barcode scanners, or digital inventory help track shelf life and movement. Once we switched to handheld scanners, losses from expired inventory dropped. Staff spotted mistakes sooner, and we proofed our operation against audits or recalls. Secure storage, accurate records, and updated software keep the supply chain safe from start to finish.
Solutions Built on Best Practices
Focusing on basics—stable temperature, low humidity, strong ventilation, active pest control, safe handling, and clear tracking—creates a safe, reliable product. Regular audits, clear training, and honest feedback from staff turn storage and handling from an afterthought to the backbone of product quality. My years in distribution and warehousing showed that these details decide which companies grow and which fall behind.
Understanding the Compound
1-Allyl-3-ethylimidazolium tetrafluoroborate stands out in the growing landscape of ionic liquids. In the lab, this salt looks almost boring — a clear or pale liquid, not very viscous, and sometimes mistaken for something mundane. Its formula grabs attention: C8H15BF4N2. Scientists label it as [AEtIm][BF4], shorthand for a molecule that's big in utility, not size.
The Important Bits: Atoms and Arrangement
Looking closer, the cation (the positively-charged half) starts with an imidazolium ring — a five-membered ring, two nitrogen atoms spaced at positions 1 and 3, then spruced up with an ethyl group on nitrogen one and an allyl group on nitrogen three. This creates flexibility in how the molecule behaves. The anion (the negative piece) is tetrafluoroborate: a boron atom huddled up with four fluoride ions. The balance of charges between the complex organic part and the sturdy inorganic tetrafluoroborate gives this compound its stability.
There’s no need for fancy 3D-simulations to see why this salt works. The way the organic cation hugs the tight little BF4- anion keeps things liquid at room temperature and blocks easy evaporation. This means a scientist shouldn’t panic about fumes or fire — which immediately reduces stress in practical settings.
Why Structure Matters Outside the Lab
Electrical engineers and chemists look for compounds that can safely carry charge or dissolve all sorts of other chemicals. This one fits the bill. That imidazolium ring, especially with the allyl and ethyl substitutions, boosts solvation power and reduces the sort of toxicity found in more basic salts. Real-world? It helps batteries run longer, lets researchers recycle metals with fewer chemical headaches, and finds its way into environmentally gentler cleaning processes.
The tetrafluoroborate group keeps things stable during intense reactions. Industrial labs rely on this for extracting precious metals and for reactions where solvents just gum up or boil off. Since the cation doesn’t like sticking to metal surfaces the way some others do, electroplating and extractions using this salt go smoother with fewer surprises. That means less downtime if you’re running a plant and healthier air for technicians working in those spaces.
Challenges and Solutions
Ionic liquids seem miraculous, but society bumps into a few problems in scaling up. One obvious issue: environmental impact. Tetrafluoroborate has high chemical stability, but spills or waste streams often contain traces. Regulatory agencies keep a close eye on fluorinated chemicals, and for good reason. To reduce worries, researchers experiment with recycling these salts, using filtration and special membranes to pull out contaminants before disposal. I’ve seen small labs work deals with suppliers to send used ionic liquids back for re-purification, cutting waste and cost at the same time.
Another issue comes with cost. Ionic liquids are pricier than the obvious solvents. It takes effort to justify buying liters of 1-allyl-3-ethylimidazolium tetrafluoroborate unless the application truly benefits from its stability or low volatility. Smaller research teams share bulk orders or partner with industry, offsetting high prices and enabling broader exploration of its uses.
Real-World Impact
Modern chemistry builds on compounds like this. They offer a smarter option for those chasing greener chemistry, longer-lasting electronics, or specialized metal processes. Examining its structure uncovers reasons for its popularity — not only how atoms link together but how those connections translate to safer, cleaner, and more efficient industry. 1-Allyl-3-ethylimidazolium tetrafluoroborate isn’t just another lab curiosity. It helps shape a future where chemistry and responsibility go hand-in-hand.
Understanding What You’re Looking For
1-Allyl-3-Ethylimidazolium Tetrafluoroborate falls under a family of chemicals called ionic liquids. These materials draw a lot of interest in labs and specialty manufacturing because they stay liquid at room temperature and offer useful electrical and thermal properties. Anyone who spends time in academic or industrial chemistry knows that sourcing exotic compounds is not the same as picking up baking soda from the grocery store.
Where to Start Your Search
Most commercial suppliers of fine chemicals put safety and legal responsibility high on their list. Companies—such as Sigma-Aldrich, TCI Chemicals, and Alfa Aesar—carry thousands of compounds, including many ionic liquids. Verification and credentials matter. Late-night orders through unknown vendors or marketplace resellers usually put the buyer at risk of low purity or scamming. No guarantee of the real thing. I stick to suppliers with clear material safety data, COAs (Certificate of Analysis), and a willingness to answer real technical questions. These companies also set clear rules: if you don’t have institutional credentials—a university or a registered business—most will not sell to you.
Why the Extra Hurdles?
I once tried sourcing a fluorinated compound for a battery project. Tons of supposed vendors online shot down my inquiries unless I could prove a connection to a research entity. Supply regulations around chemicals like 1-Allyl-3-Ethylimidazolium Tetrafluoroborate don’t just come from suppliers protecting their reputation. National and regional law (like REACH in Europe or TSCA in the United States) sets boundaries on sales of anything with environmental or misuse risks. This level of caution protects people and ecosystems from improper use or disposal, and it limits the chances of someone trying to skirt rules for nefarious purposes.
How the Process Usually Works
Let’s say you work in a university lab. The purchasing department comes into play. They’re the team authorized to interact with reputable chemical companies. Most suppliers run a documentation check before they even send a quote. They almost always ask for an end-use statement explaining the purpose for buying the material. This helps to confirm you don’t plan on using the compound for anything outside of ethical or legal research. Only after account approval do lists of available packaging sizes and prices become visible. I’ve only ever seen small samples—maybe 1 or 5 grams—offered without a full account. Large-quantity sales bring more scrutiny.
Spotting Red Flags
Platforms that claim overnight shipping with no questions asked signal trouble. Ads with little technical detail or missing regulatory documentation raise real concerns. I have seen plenty of dubious offers on message boards and even through email spam. Many offer “lab supply” without a traceable address or phone number. These options run contrary to both scientific best practice and safety culture. Not only do you risk wasting money, but mishandling certain chemicals endangers both the buyer and the community.
Responsible Sourcing Moves Science Forward
For anyone passionate about chemistry, sourcing with care goes beyond just ticking off procurement boxes. Sticking to trustworthy vendors with clear legal standing protects both people and reputations. It also means labs stay in step with global environmental responsibility. Real innovation happens only when you know the materials on your bench match what’s printed on the label—and that’s something no shortcut can replace.