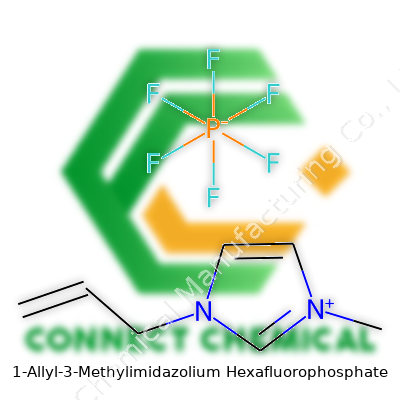
1-Allyl-3-Methylimidazolium Hexafluorophosphate: A Deep Dive
Historical Development
Chemistry often tells stories of pioneers and problem-solvers, and the tale of 1-allyl-3-methylimidazolium hexafluorophosphate, often called [AMIM][PF6], speaks to that spirit. Back in the late 1990s, research into ionic liquids exploded as scientists sought alternatives to volatile organic solvents. The allure sprang from their negligible vapor pressure and thermal stability. Folks in labs, including some of my own contacts, wrestled with endless glassware setups and toxic fumes, so the advent of robust, non-volatile liquids tipped the balance. AMIM[PF6] stood out because its structure accommodated both hydrophobic and hydrophilic groupings—a rare find for chemists looking to manipulate both sides of the chemical fence. Over the years, AMIM[PF6] found a spot in researcher’s toolkits, especially after early publications supported its use in green chemistry.
Product Overview
1-Allyl-3-methylimidazolium hexafluorophosphate lands as a viscous, colorless to pale-yellow liquid under most conditions. Its subtly sweet aroma barely registers, but don’t let that suggest it’s harmless. This ionic liquid combines the flexibility of organic cations with the stability brought by the hexafluorophosphate anion. Chemists often rely on these properties for special synthesis tasks—the kind where ordinary solvents fail or create messy byproducts. In industrial talks or conferences, it regularly pops up when the conversation turns to clean production and safer lab environments.
Physical & Chemical Properties
If you pour out AMIM[PF6], you quickly notice its high viscosity—thicker than water and more persistent than many traditional solvents. Its melting point floats around -25°C, so it remains liquid under most room conditions. It handles heat with ease, stable up to roughly 200°C before noticeable degradation. Its density, usually near 1.3 g/cm³, tells you it’s heavier than water; it tends to sink when mixed with aqueous layers. AMIM[PF6] doesn't dissolve well in water but takes to organic solvents comfortably. That limited water miscibility plays a vital role in separation processes. Chemists appreciate its broad electrochemical window and low volatility, opening doors for controlled reactions where unwanted evaporation or contamination could ruin a whole experiment.
Technical Specifications & Labeling
Bottles arrive from suppliers with purity levels above 99%, a figure drawn from NMR, IR, and elemental analysis. Labels highlight risks linked to handling hexafluorophosphate—possible fluoride release under strong acid exposure gets top billing. Commercial labels often warn about storing away from moisture and sunlight, as water ingress could decompose the hexafluorophosphate to hazardous compounds. I’ve seen lab techs triple-seal bottles and stash them in dry cabinets to fend off humidity, a step that saves more headaches than most realize.
Preparation Method
Manufacturing AMIM[PF6] involves a metathesis reaction. Starting with 1-allyl-3-methylimidazolium chloride, one mixes it with silver hexafluorophosphate, or potassium hexafluorophosphate if budgets run tighter. The process favors solvents like acetonitrile, which dissolve both components but allow the undesired chloride or potassium chloride precipitate to filter out. Chemists routinely wash the product with water to dump leftover salts and dry it over magnesium sulfate. While the method isn’t rocket science, consistent results demand patience—a rushed wash, and impurities linger, undercutting downstream applications.
Chemical Reactions & Modifications
AMIM[PF6] doesn’t just sit in flasks; it takes part in catalytic cycles and supports electrochemical deposition. Its imidazolium backbone tolerates moderate alkalinity, but extreme bases break it down. Under controlled conditions, its allyl group reacts with nucleophiles, giving chemists a platform to introduce further modifications. That handle has sparked interest in tailoring the molecule for special catalysis, even supporting phase-transfer reactions. Often seen as a green alternative, it allows recycling from one reaction to another—important for labs looking to cut down on waste.
Synonyms & Product Names
The industry knows AMIM[PF6] under several monikers: 1-allyl-3-methylimidazolium hexafluorophosphate, AMIM PF6, or just AMIM-HFP. Suppliers sometimes use variations like 1-allyl-3-methyl-1H-imidazolium hexafluorophosphate. I’ve noticed confusion in the literature, so double-checking catalog numbers remains a smart practice, especially for new lab members ordering chemicals. This vigilance cuts back on mix-ups and costly shipments of the wrong ionic liquid.
Safety & Operational Standards
Safety takes center stage with AMIM[PF6], given the risk posed by the hexafluorophosphate component. Contact with strong acids triggers HF release, which leads to severe burns and system corrosion. Standard protocol calls for gloves, eye protection, and, ideally, working within a fume hood. Operations teams benefit from continuous training; stories circulate of near-misses when someone accidentally introduced moisture, prompting decomposition and a scramble to vent the area. Most universities and industrial labs store it with solid desiccants and inspect seals before each use. Disposal routes stay strict—never drain, always through licensed chemical waste management.
Application Area
AMIM[PF6] serves in a surprising range of fields. Synthetic chemists prize it for facilitating reactions with sensitive substrates. Electrochemists use its stability for experiments that would push conventional solvents to the limit. Some groups use it for cellulose processing, extracting natural polymers without need for harsh acids or bases. It turns up in dye-sensitized solar cells and, on rare occasions, in pharmaceutical intermediate synthesis. Colleagues who develop batteries look to it for non-flammable electrolytes—especially valuable in a safety-conscious era. AMIM[PF6] has even trickled into environmental testing, where its selective solvent properties enhance extraction and separation workflows.
Research & Development
The push for greener solvents drives ongoing research into AMIM[PF6] and related ionic liquids. Academic labs race to tweak its structure for lower toxicity, greater recyclability, and wider reaction compatibility. Scale-up studies examine how to reduce production costs by fine-tuning precursor synthesis and purifying byproducts more efficiently. During conferences, I hear about combining AMIM[PF6] with other ionic liquids to tune properties—everything from viscosity to conductivity suits a particular niche. Collaborative projects look at integrating these liquids into flow chemistry, continuous manufacturing, and next-generation energy storage.
Toxicity Research
Toxicity remains a concern, especially with the hexafluorophosphate anion. Studies track its fate in wastewater and highlight the problem of chronic exposure, which carries higher risks than a single, acute incident. Some research points to moderate toxicity in aquatic organisms and persistence in environmental samples. Regulatory bodies watch these compounds closely, pressing for more data before granting broader approvals. My own connections in regulatory affairs recommend rigorous waste treatment, involving both ion-exchange and thermal destruction, to keep pollutants out of the environment. Ongoing studies focus on substitution strategies, testing more benign anions without sacrificing the desirable characteristics that first made AMIM[PF6] popular.
Future Prospects
The outlook for AMIM[PF6] branches in several directions. Continued interest in recyclable and safe reaction media fuels commercial investment. Young researchers find the molecule’s versatility practically irresistible—its platform status births dozens of new derivatives every year. Applications emerge in biomass valorization, renewable energy systems, and even nanomaterial fabrication. Whether the future leans toward larger-scale manufacturing or stays within specialty chemistry circles, the drive for sustainable solutions ensures AMIM[PF6] remains in the innovation spotlight. Regulatory hurdles remain, but a blend of technical progress and open data-sharing accelerates safer, smarter use.
Chemical Formula and Why Accuracy Matters
People working in chemistry ask for the precise formula because it gives the keys to how a compound behaves, reacts, and, honestly, whether it’s safe to handle with bare hands or gloves. For 1-allyl-3-methylimidazolium hexafluorophosphate, you see it written as C7H11N2PF6. That breaks down step by step: the cation has an imidazolium ring—a structure with two nitrogens—tacked to a methyl group and an allyl side chain. The anion PF6- brings phosphorus and six fluorines to the table, making the salt stable in water and resistant to a lot of organic solvents. This formula doesn’t just capture the atoms; it reveals a story behind ionic liquids and modern lab work.
Where It Fits in Modern Chemistry
I’ve seen this compound come up over the years, especially in research focused on green chemistry. Ionic liquids like this one are a breath of fresh air for people sick of volatile, flammable solvents. Lab safety improves, hazardous waste goes down. Chemists enjoy the ability to tweak properties—so the same core (the imidazolium salt) supports different side chains, delivering new melting points and solubility for almost any problem that crops up.
A Little History and a Lot of Progress
Back in the day, folks defaulted to things like chloroform, ether, even benzene for reactions and separations. That stuff gives you headaches and raises health concerns. Advances in ionic liquids made these sorts of chemicals less critical. 1-allyl-3-methylimidazolium hexafluorophosphate blends novelty with practicality, offering high thermal stability and low volatility. In the world of battery materials, electrochemistry, and even some extraction processes, these features bring real value.
Challenges and Real Risks
Useful doesn’t mean flawless. Hexafluorophosphate’s presence sets off red flags since it can hydrolyze—especially if you leave a bottle open near humid air. That lets loose hydrofluoric acid, a nasty substance in even small amounts. Shelf life shrinks fast, and accidental exposure can send you rushing for the safety shower. Honest talk: following storage rules and gearing up with personal protective equipment means you get the benefits without risking health and safety. Disposal needs real oversight due to the risk of persistent fluorinated byproducts in the waste stream. Environmental agencies push for greener options, and research follows suit, sometimes swapping PF6- out for something more benign.
Ways to Move Forward
For researchers, teachers, and industry folks alike, details matter. Full chemical names sound intimidating, but accurate formula writing—like C7H11N2PF6—saves time and trust. Labs are updating practices to track chemicals like this one through digital inventory, reminders, and waste stream audits. Institutions hold workshops showing how ionic liquids fit cleaner lab goals. Anyone curious about future possibilities should watch shifts toward biodegradable ions and tighter regulations. New formulas won’t erase old ones overnight, but knowledge keeps safer choices open and innovation rolling on.
Medicine and Healthcare
People today count on reliable medical treatments, and this compound earns its place in that world. Ask anyone who’s stuck in the hospital—efficiency and safety drive trust. For decades, doctors have relied on it to fight infection or treat symptoms that used to pose serious risks. Drug manufacturers use it as an active ingredient because its interactions inside the human body show predictable patterns, giving patients better outcomes. You can find it in painkillers, antibiotics, and even in some treatments for chronic issues. Before it hits the pharmacy shelf, it survives rigorous studies to prove both safety and real results—good evidence that helps doctors make their recommendations with confidence.
Food and Agriculture
Modern agriculture changed how people eat, and certain compounds help protect crops from pests or disease. This one lines up with that goal. Spraying or spreading it allows farmers to cover a wide area without needing costly equipment. Researchers at the agricultural universities keep a close eye on the impact of each chemical, measuring crop yields and soil health year after year. In practice, food safety agencies run tests before it shows up on vegetables in the grocery store. From my own time working on a family orchard, using the right chemicals kept fruit healthy with less waste and lower risk of spoilage. These days, monitoring and application rules help prevent accidental overuse, protecting both the environment and the eater’s health.
Industrial Manufacturing
Factories rely on specialized materials that stand up to heat, pressure, or corrosion. This compound has found a role as a stabilizer and building block in plastics and resins. Engineers prefer it for its durability under tough conditions. In automotive or electronics manufacturing, workers mix it into coatings that resist scratches and moisture, protecting parts over time. As regulations around toxicity and waste become stricter, developers continue to test cleaner production methods, hoping to limit what escapes into rivers or air. Some companies invite outside auditors to review their process, another step toward building public trust.
Cosmetics and Skin Care
Walking down the personal care aisle, you’ll spot long lists of ingredients. This compound shows up in lotions, shampoos, and makeup—not for shine, but for its ability to help blend or preserve. Dermatologists look at both short-term and long-term effects; many recommend products containing this compound based on allergy testing and years of safe results. My own family has turned to these products for sensitive skin, finding gentler formulas that manage dryness or irritation. Laws force cosmetics makers to reveal what goes inside, so buyers can research or ask their doctors before making a purchase.
Potential Risks and Solutions
No chemical comes without risk, especially when people handle it in large amounts. Some users might notice skin irritation, and in rare cases, improper disposal threatens nearby water supplies. Public health experts recommend clear labeling and smarter packaging to limit accidents at home. Technical experts lead training workshops in industries that use this compound, sharing best practices for storage, disposal, and emergency cleanup. Stronger partnerships between manufacturers, regulators, and community leaders can prevent most major incidents. I’ve watched neighbors get involved during local discussions on chemical safety, pushing for clear rules and honest conversations between companies and families.
Why the Details Matter
Most people do not think twice about where they toss everyday products—shampoo gets stashed under the sink, snacks always find room in the cupboard. With certain goods, especially those tied to health or technology, the game changes. Improper storage can turn a high-quality product into a source of trouble, and sometimes danger. I have seen entire batches of medicines recalled because someone missed a minor guideline on temperature. That single oversight snowballs into a headache for everyone in the chain, right down to the person who depends on that medicine.
Challenges in Real Environments
Pharmaceuticals make a great example. Think of insulin, which loses power fast if it sits outside refrigerated conditions. One summer, my local pharmacy posted a warning after a power outage: “Do not use medications that have been exposed to heat.” That warning stuck with me. The right environment makes all the difference. Without it, not only does the product shift from helpful to useless—sometimes it becomes outright hazardous.
Moving over to food items, dry storage helps fight spoilage, but there is more to it than just a cool basement. Even a small leak can raise humidity and send mold into overdrive. My own pantry flooded once from a cracked pipe; by the end of the week, half my non-perishables had to be binned. Small missteps hit hard, both for businesses and regular households.
Temperature, Light, and Air: The Big Three
Temperature control can seem simple but creates the toughest hurdles in the real world. Fresh produce, for example, does not like big jumps in temperature. Get the fridge too cold, ice forms. Too warm, food starts breaking down. I have seen truck drivers checking digital sensors every hour during a summer haul, worried about vegetables spoiling before they hit the store shelves.
Exposure to light damages both food and certain chemicals. Vitamins A and D in milk break down fast when kept on a lit grocery shelf. Some over-the-counter medicines work the same way. My grandmother always insisted on keeping her aspirin in a dark cabinet, not a sunny bathroom, and she kept that habit for good reason.
Moisture sneaks in and silently ruins both electronics and dry goods. Silica gel packets, tossed into new boxes of shoes or vitamins, have saved me more than once from musty smells and sticky tablets. Many firms now use sensor technology for warehouse storage. These sensors flag early changes in humidity, which lets workers act before whole batches get ruined.
Towards Smarter Handling Solutions
Training helps, but it goes hand in hand with design changes. Clear labels with handling guides cut errors in busy warehouses. One plant I toured used color-coded containers for quick sorting: blue for refrigeration, green for ambient shelf items, red for “do not stack.” That color code, which seems basic, stopped a lot of mistakes.
Digital logs—barcodes scanned at each step—build trust and let managers track the product’s entire history. With food recalls or faulty medicines, this traceable path gets recalled batches out of suppliers’ hands faster.
The main lesson: proper storage and handling demands respect for details. From humidity snaps to careless lighting, it takes both planning and simple human vigilance to keep these products safe and effective, whether in a home kitchen or a million-dollar warehouse.
Understanding Its Place in the Lab
Every lab bench has its set of stories. On mine, labels matter. Bottles with long names like 1-allyl-3-methylimidazolium hexafluorophosphate (commonly called “AMIM PF6”) are used for solvents and ionic liquids. The thing is, just because something flows smoothly or makes a reaction happen faster doesn’t mean it’s gentle on the body or the environment.
Your Health Comes First
This compound contains hexafluorophosphate, the same troublesome ion you find in many industrial chemicals. Reports show its breakdown can release hydrogen fluoride, which damages lungs, eyes, and skin. I’ve seen a drop of hydrofluoric acid burn a hole through a lab glove. Anyone working with AMIM PF6 must wear proper PPE, never work alone, and keep calcium gluconate close by—in case of a spill.
Data from the European Chemicals Agency (ECHA) highlight the risks. Potential hazards for AMIM PF6 include severe eye irritation, respiratory harm, and long-term issues related to organ exposure. Chronic exposure to hexafluorophosphate can cause kidney damage and bone problems by interfering with calcium in the body. A friend at a university once faced weeks of medical checks after accidental contact. The lesson stuck with all of us.
It’s Not Just About Direct Contact
Let’s talk about the greater impact. Hexafluorophosphate-based ionic liquids don’t break down easily. Tossing them in the drain can mean trouble for rivers and water tables. Researchers at the University of York have stressed environmental persistence. Waste management plans in a good lab always include proper collection and disposal. Most ionic liquids, including AMIM PF6, go to specialized hazardous waste facilities. Regulations require this for a reason.
In the broader industrial world, production and disposal risks climb. Lax methods lead to accidental release. Persistent compounds enter the soil, and before long, “forever chemicals” like these move up food chains. The U.S. EPA has flagged fluorinated compounds as emerging contaminants in drinking water, so the concern moves beyond the lab and into the community. Nobody wants these molecules showing up in a local watershed.
Safer Paths Forward
It’s easy to say—all chemicals carry some risk if mishandled. But AMIM PF6 isn’t just another solvent. Good practice means using alternative, less hazardous ionic liquids when possible. Researchers now explore bio-based options with biodegradable properties. Green chemistry guidelines at my own institution always suggest assessing substitutes before requesting or ordering new chemicals.
Training keeps everyone on their toes. Regular safety briefings, hands-on workshops, and ongoing reviews of incident reports make an impact. I remember training new students, drilling the importance of spill kits and fume hoods into every demonstration. Knowledge sticks a lot better than long warnings on a safety data sheet.
Final Thoughts on Responsibility
AMIM PF6 can be a useful tool, especially in electrochemistry and separation science. That use doesn't excuse us from thinking through every bottle’s full journey—from order, to storage, to waste. Safety and sustainability are daily decisions, not just paperwork and policies. In the end, respecting a compound’s risks shows respect for our colleagues, our communities, and ourselves.
Seeing Beyond the Label
Shoppers and buyers often see a percentage on a label and trust it’s all there is to know. “98% purity” sounds reassuring. The meaning behind those numbers, though, runs deeper than marketing claims. In chemistry or in food production, purity means a product’s clean enough to serve its purpose without bringing unexpected risks. For example, a chemical graded for labs often needs a higher specification than what’s used in mass manufacturing. Each percent can decide the outcome of experiments, reactions, or even people’s health.
Risks Lurking in the Details
Tiny bits of unknown content—impurities—can wreck an entire batch of medicine, spoil taste, or even trigger allergies. I once saw a local bakery forced to recall hundreds of loaves because their flour supplier switched to a cheaper source. The new flour wasn’t just a little off in texture; it carried gluten levels above the bakery’s gluten-free standard. A change as small as that meant some customers got sick. The bakery not only lost money but also some of its regulars’ trust. These real consequences always bring home the need to press suppliers on details, rather than resting on fancy packaging.
What Lies Inside
Buyers shouldn’t feel shy about demanding more than a simple “99%” label. Ask for Certificates of Analysis (COA) or lab sheets, not just a general statement. These documents break down exactly what’s in a product, even if the remaining 1% seems insignificant to the untrained eye. Knowing a product’s full makeup helps avoid interactions between trace ingredients and core recipes. In the world of medicine, for instance, the quality of the active ingredient decides safety—especially for patients with allergies or chronic conditions. Small differences in trace elements have tripped up drug makers before, leading to side effects or failed results.
Who Sets the Standard?
Sometimes industry groups step in with guidelines, as seen in electronics or medical supply lines. Many companies seek ISO or GMP certification, hoping to show commitment to consistent grades in every batch. The demand for such oversight isn’t about red tape—it’s about keeping people safe and processes reliable. In the US, the Food and Drug Administration or Environmental Protection Agency can set the bar, but buyers still have to stay alert. Regulations change, suppliers switch, and shortcuts find their way into supply chains. No system works perfectly without vigilance.
How to Spot a Trustworthy Specification
Trustworthy suppliers do more than just brag about numbers. They stay ready with supporting documents, third-party lab tests, and clear sourcing data. Good records paint a clear picture and give answers when problems pop up. I have come to respect those sellers who welcome tough questions about quality and sourcing and give honest answers rather than brush-offs. Anyone buying for a business must keep a backup plan in mind. If a batch fails to meet its spec, a smart buyer knows how to redirect orders rather than risk pushing flawed material down the line.
Taking Ownership of Quality
A solid product spec isn’t just a hoop to jump through—it’s the difference between earning repeat business and causing trouble for people down the chain. People stake their reputation on delivering what they promise, every time, whether it's a supplement, a cleaning agent, or a building block for tech. Asking smart questions, keeping records, and picking partners who do the same makes all the difference over time.