An Article on 1-Allyl-3-Methylimidazolium Tetrafluoroborate: Background, Properties, and Future Potential
Historical Development
Back in the late 1900s, chemists started thinking differently about solvents and salts. Environmental concerns and tougher regulations drove scientists to look for ways to replace traditional, often toxic, solvents. While looking for alternatives, researchers discovered that some salts could stay liquid at room temperature. These salts, known as ionic liquids, transform our understanding of how chemical separations and syntheses can work. 1-Allyl-3-methylimidazolium tetrafluoroborate, a mouthful for sure, didn’t take long to emerge from that burst of creativity. By the late 1990s and early 2000s, it had become a favorite for experimentation in labs tackling task-specific ionic liquid design.
Product Overview
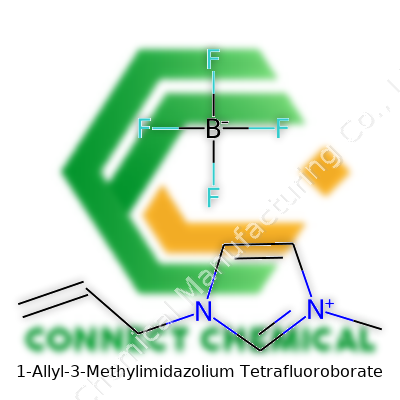
1-Allyl-3-methylimidazolium tetrafluoroborate comes up regularly in research settings. People usually shorten its name to [AMIM][BF4] or sometimes call it AliMeIm-BF4. This chemical belongs to a growing family of imidazolium-based ionic liquids, which show up as colorless or pale yellow liquids. You can spot scientists pouring it straight from a bottle into vials for synthesis, or dissolving metals or cellulose with it. Teams working on new battery technology or exploring enzyme stability keep bottles of this stuff on their shelves, impressed by how it can thrive in places where traditional solvents break down or cause problems.
Physical & Chemical Properties
You notice right away that [AMIM][BF4] doesn’t behave much like water or other liquids most folks are familiar with. It’s remarkably stable, barely evaporates, and keeps its cool across a wide temperature range. It also refuses to catch fire under most conditions, which cuts down on panic during lab accidents. Its density settles between 1.1 and 1.2 g/cm3, and it carries an impressively low volatility. The melting point averages below room temperature. Electrically, it’s a decent conductor—for an ionic liquid—which makes it popular in electrochemical devices. It dissolves a surprising number of organic and inorganic materials, opening new doors that water or acetone can’t unlock.
Technical Specifications & Labeling
Companies package this ionic liquid with attention to moisture. Air and water slowly turn it sour, so tight-sealing amber vials do the trick. Labs check for color, clarity, and sometimes purity using NMR and Karl Fischer titration. Labels note the exact composition, with a CAS number of 171058-18-7 often being the most recognizable string of digits. Volume and weight get measured carefully, as even a tiny contamination changes its behavior. Shelf-life sits squarely on storage conditions, but you can typically expect well-sealed samples to last through dozens of experiments, keeping their sparkle.
Preparation Method
Making this stuff from scratch takes patience. Most routes start with methylimidazole and allyl chloride, letting them react in a controlled setting, then neutralizing the intermediate salt with sodium tetrafluoroborate. Purification follows, requiring vacuum drying and repeated washes with ethyl acetate or other cleaning agents. Students and industry chemists often tweak details based on desired purity, but the core chemistry stays simple enough for a well-equipped lab. I’ve watched colleagues run these syntheses, logging temperatures and stirring speeds to replicate published yields, always hunting for traces of unreacted materials with TLC or NMR.
Chemical Reactions & Modifications
Standing out for its reactivity, [AMIM][BF4] draws attention for both its own chemistry and the chemistry it enables. The allyl group attached to the imidazolium ring can take part in further reactions, forming new polymer networks or immobilizing onto solid surfaces. The BF4- anion sometimes gets swapped out to adjust the ionic liquid’s physical traits for custom tasks. Chemists graft additional functional groups onto the imidazolium core, expanding its reach into catalytic cycles or selective binding environments. At the bench, modifying this ionic liquid often means better viscosity control, new charge transport properties, or capturing specific solutes more efficiently.
Synonyms & Product Names
This compound’s lengthy chemical name rarely rolls off the tongue, so industry and research labs rely on shorthand. [AMIM][BF4] is the common go-to, with other labels showing up in catalogs, including AliMeImBF4, 1-allyl-3-methylimidazolium fluoroborate, and variations like 1-allyl-3-methylimidazolium tetrafluoroborate. Each name points to the same core structure, helping researchers recognize the compound in literature or product databases.
Safety & Operational Standards
No matter how useful a chemical looks, safety trumps all else when working with unknowns and new materials. [AMIM][BF4] rarely sets off alarms like acids do, but sharp attention to glove use, fume hoods, and splash protection goes without saying. Trace water absorption or decomposition sometimes produces acids, so I’ve seen chemists keeping an eye on pH just to be sure. Waste disposal involves keeping spent material apart from regular solvent waste, as some tetrafluoroborate compounds release fluorine-containing products under heat or UV. Regulatory guidelines continue to evolve, and folks in industry track updates closely, aligning storage, shipping, and use with best practices as more is learned.
Application Area
Over the past decades, fields tapping into ionic liquids explode in number and variety. In my own experience, battery and capacitor researchers light up discussing the conductivity and electrochemical stability [AMIM][BF4] brings to new designs. It helps dissolve stubborn cellulose for biofuel work, and enables smoother organic syntheses, especially for challenging separations. Catalytic cycles in green chemistry push forward thanks to its unique properties. Enzyme stabilization, gas absorption, solar cell fabrication—the list grows every year. You see it referenced everywhere from academic journals about pharmaceutical synthesis to patents for advanced lubricants.
Research & Development
The sense of energy in labs looking at ionic liquids stays palpable. Research teams keep refining ways to tweak [AMIM][BF4], whether by changing the anion, grafting new functional groups, or blending it with biodegradable additives. Journals regularly feature studies comparing its ability to separate metal ions or stabilize biomolecules with other, more established ionic liquids. On the industrial side, pilot-scale reactors now exist for producing this compound at kilogram scales, which felt like a far-off dream just a few years ago. From my own observations, as synthesis matsures, research pivots more toward lifecycle analysis and recovery, recognizing that even green solvents need responsible stewardship at scale.
Toxicity Research
You can’t claim a chemical is green without proving its impact on people and environments. Researchers track toxicity profiles for every ionic liquid, including [AMIM][BF4]. Studies highlight that it generally doesn’t vaporize or carry acute risks, yet questions linger about long-term aquatic toxicity and soil persistence. Cellular assays measure its effect on common model organisms, with some vigilance needed as subtle impacts sometimes show up after several generations or under stress. To me, that means progress relies on honest, rigorous reporting and cross-checking toxicity results, especially as more industries investigate its use.
Future Prospects
Looking ahead, the landscape keeps shifting for ionic liquids like [AMIM][BF4]. Breakthroughs in battery and CO2 capture often mention this compound as central to the next wave of technology. Bio-based feedstocks for ionic liquid production attract investment, promising smaller environmental footprints and greater scalability. Recyclability programs, led by both industry and academia, focus on closing the loop and minimizing waste. By taking what’s learned from demonstration-scale projects and feeding it back into design, the field keeps moving closer to genuinely sustainable chemistry. It feels like every few years, a new application redefines the boundaries. The story of [AMIM][BF4]—from niche lab curiosity to a genuine workhorse—deserves close attention as the world seeks safer, cleaner, and smarter solutions across science and industry.
More Than a Chemical Name
Plenty of people outside the lab walk right past complicated chemical names like 1-Allyl-3-Methylimidazolium Tetrafluoroborate without realizing how much this stuff matters. Scientists and engineers have gotten creative with it because of its set of traits: it doesn’t evaporate easily, it dissolves lots of things, and it has a knack for carrying electric charge in the right situations. Those are big pluses. I’ve watched researchers light up when they swap out traditional solvents for this ionic liquid during their projects.
Green Solvents and Cleaner Processes
Old-school solvents often harm the air, water, and their users. Some even stick around in landfills or nature for years. I picked up on this problem years ago, and many labs started switching over to “greener” options. That’s where 1-Allyl-3-Methylimidazolium Tetrafluoroborate steps in. It works in processes where you want to clean up your act, literally and figuratively. It breaks down cellulose with more control, and chemists like extracting bioactive compounds from plants and pharmaceutical leftovers without creating nearly as much toxic waste. Making things safer isn’t just hype; this lets big companies and universities lower their emissions and lessens headaches for workers who used to deal with tougher smells and spills.
Electrochemistry’s Secret Ingredient
Look at batteries, fuel cells, and even fancy sensors. Many of these technologies run into limits because of the messiness or dangers of traditional liquids used to move ions around. 1-Allyl-3-Methylimidazolium Tetrafluoroborate doesn’t just carry a charge—it holds up under long tests, and it keeps working at temperatures where others fail. I remember seeing test runs where standard solvents boiled off or broke down, while this liquid stuck around. The push for better, safer supercapacitors and flexible batteries in electric vehicles keeps this ionic liquid in the spotlight.
Speeding Up Chemical Reactions
Chemists used to wait hours or days for some reactions to finish. Shake up the mix with something like 1-Allyl-3-Methylimidazolium Tetrafluoroborate, and things go faster. Because it doesn’t evaporate much, it concentrates what you want to react, making the reaction move along quicker. I’ve watched students trim hours off their experiments, saving energy bills at the same time. Industrial production lines feel that kind of time savings even more; it means higher output with less waiting around.
Solutions That Last Beyond the Lab
Outside pure chemistry, this ionic liquid starts showing up in material science and environmental clean-up. Researchers blend it with nanomaterials, searching for tougher coatings and new types of membranes. In waste treatment, it pulls out heavy metals from water more efficiently than stuff we used before. For those of us tracking water safety, that’s a game-changer, especially in places with aging infrastructure.
Looking Forward
Any tool with this much promise still faces hurdles. Scale-up can run into cost problems, and not every industrial plant feels ready to ditch old setups. There’s plenty to figure out, like how to recycle or reuse the liquid after use. Teams working on cheaper versions and new recovery methods keep making progress. From what I’ve seen, the drive to clean up manufacturing, improve batteries, and reduce waste means 1-Allyl-3-Methylimidazolium Tetrafluoroborate won’t stay a niche ingredient for long.
Everyday Risks and Practical Lessons
Every chemical on the shelf carries its own story, sometimes easy, other times lined with serious side effects. I’ve worked in labs and seen the mess a forgotten cap can cause. Spills eat through countertops, fumes soak into drywall, and what seems trivial at first can escalate fast. Let’s get real: safe storage and handling demand a sharp eye, steady habits, and a deep respect for the substance in question.
Common Sense Outweighs Fancy Labels
Labeling can help, but a label alone won’t block a chemical from reacting with the wrong neighbor. Acids and bases bicker in close quarters. Oxidizers don’t get along with organics. Keeping sodium hydroxide away from hydrochloric acid stops a toxic cloud from forming. The only foolproof plan links careful segregation with straightforward organization. It helps to separate flammables, corrosives, oxidizers, and toxins with a clear system. Simple shelves and secondary containment pans, cleaned regularly, lower the odds of a disaster no warning label can explain.
The Truth About Temperature and Ventilation
Heat and fumes combine to form an invisible threat. Some chemicals will cook up extra pressure if storage temperatures spike, especially in unventilated closets. That’s a recipe for bottles bursting or vapor leaks. Flammable solvents, for example, demand cooler conditions in steel safety cabinets. Load up the cabinet, but don’t cram it full—air needs to move. I’ve felt the whoosh when a too-warm room blasts chemical odors your way. That’s always trouble.
PPE: The First, Last, and Only Real Line of Defense
People skip safety gear, thinking a splash won’t happen to them. Goggles fog up, gloves feel clumsy, aprons get tossed aside during cleanup. From experience, that’s courting trouble. One tiny spill of concentrated acid, and your skin or eyes will send the lesson home. Training on gear can get routine, but it’s essential. Even the most experienced handler can get complacent without reminders. Proper gloves, eye shields, and sometimes respirators provide more than peace of mind—they give a fighting chance if mistakes happen.
Regulations: A Floor, Not a Ceiling
Rules from OSHA, NIOSH, and EPA don’t just exist for paperwork’s sake. Federal guidelines lay out fire limits, secondary containment, and spill response so nobody needs to invent best practices on the fly. Yet, rules count for little unless put into action. Inventory checks keep surprises out. Inspection logs force accountability. Locks and sign-in sheets stop unauthorized access. Most incidents I’ve seen came from cutting corners, not lack of information.
Solutions Rely on Culture, Not Just Protocol
Strong storage and handling start with clear habits and shared responsibility, not just posters on the wall. Training sessions pay off only if every person feels empowered to act. Staff should fix misplaced containers, speak up about leaks, and shut the storeroom if vent fans sputter. Leadership steers the ship—a manager who champions safety and models the steps encourages everyone to follow. Regular drills, honest incident reviews, and a climate of respect for risk make every other precaution work.
Practical Takeaways
The science behind chemical safety isn’t hidden in thick manuals. Store chemicals by hazard class, manage temperature and airflow, lock up what can do harm, and don the gear every time. Most of all, remember: one mistake can outweigh a hundred safe days, but it takes only a nudge in culture to make safety routine instead of a chore.
Looking at the Science
1-Allyl-3-methylimidazolium tetrafluoroborate belongs to the class of ionic liquids. Thicker than water and almost slippery like oil, these liquids dissolve salts and run much cooler than many solvents you find in a lab. Chemists from the early 2000s buzzed with excitement about ionic liquids because they don’t evaporate into the air and catch fire easily. In industrial settings, this kind of liquid promises less waste and lower energy bills. On paper, these properties look like a future-proof answer to cleaner chemical processes. But cleaner does not always mean safer.
The Flipside of Low Volatility
Skip the lab coat for a second and check the facts: you don’t want this stuff on your skin. Ionic liquids like 1-allyl-3-methylimidazolium tetrafluoroborate can irritate eyes, corrode skin, and might even damage organs over time if you breathe in droplets or spill it often. A recent study published in Chemosphere showed that some imidazolium-based liquids mess with aquatic life, especially tiny water fleas and fish larvae. Most ionic liquids barely degrade in water, so when a spill runs down the drain, it lingers. This means more risk to rivers, not less.
Comparing to Common Solvents
Plenty of solvents—acetone, hexane, toluene—are toxic in their own ways. Laboratory staff and chemical handlers wear gloves and goggles for every drop. But some industries have tried to swap out those classic solvents for ionic liquids hoping for lower fire risk and less smog. The punchline? If you switch to something new, better check every angle. Studies from the European Chemicals Agency mark 1-allyl-3-methylimidazolium tetrafluoroborate as an irritant. It can be absorbed through the skin. If you breathe the vapors or fine mist, it could damage your upper airway and lungs. So calling it “green” ignores the full story if cleanups and spills aren’t managed right.
Toxicity in the Real World
There’s a bigger gap than people expect between “not flammable” and “not toxic.” My own time in academic labs taught me respect for all unknowns in a bottle—especially ionic liquids. Safety data sheets spell out risks: this chemical can harm aquatic ecosystems, make skin blister, and cloud your eyes if you splash it. I have seen students treat ionic liquids with less care because they don’t smell harsh. That’s risky thinking. The molecules might sneak right through nitrile gloves. We reached for thicker gloves for a reason.
Toward Safer Use
Companies and lab techs can do more to protect workers and the environment. Chemical handlers should double up on gloves, keep spills off floors, and use goggles every time. Fume hoods and splash shields reduce exposure to skin and lungs. Wastewater treatment needs tighter controls before anything washes away from labs or plants. Governments need quicker data on emerging chemicals—don’t wait for disaster before running the tests. I’d call for an update to safety training wherever these liquids enter the scene.
Balancing Progress
Calling 1-allyl-3-methylimidazolium tetrafluoroborate safe or hazardous misses the point. No miracle solvent solves every risk. Science and industry both need honest conversations about trade-offs. If companies want to use this liquid, everybody—students, workers, regulators—must respect the need for strict rules and open information. This isn’t just a decision about chemistry. It’s about putting health and real “greener” practices above buzzwords.
Getting to the Heart of Ionic Liquids
Ionic liquids have sparked interest because of their unusual characteristics. These compounds stay liquid at room temperature, defying what most expect from salts. The trick lies in their chemistry. Classic examples combine bulky organic cations with inorganic or organic anions, creating salts that don’t stack well and resist crystallization.
Diving Into the Structure
One widely known ionic liquid is 1-butyl-3-methylimidazolium hexafluorophosphate. The cation—1-butyl-3-methylimidazolium—features a five-membered imidazole ring, linked to a butyl group at one nitrogen and a methyl group at the other nitrogen. The anion, hexafluorophosphate (PF6−), comes from phosphorus at the center, surrounded by six fluorine atoms. This pairing keeps the liquid phase stable at low temperatures.
Looking at the actual molecular formula, the cation shows up as C8H15N2+. The anion reads as PF6−. When these come together, you find the formula written as C8H15N2PF6.
Why Care About These Components?
Understanding the structure is not just for chemical trivia. That bulky cation, for example, has an impact on many things, like viscosity and solubility. Longer chains on the cation help shift ionic liquids from being slightly oily to outright greasy. The anion can turn the whole liquid water-friendly or water-hating. PF6− keeps ionic liquids hydrophobic, making them useful for non-aqueous chemical work.
Chemists and engineers need to grasp these details so they can match an ionic liquid to a job. For example, someone in a fuel cell project picks different ionic liquids than someone cleaning up metal waste from electronics recycling. The hexafluorophosphate anion comes with its own set of pros and cons: it resists water and oxygen attack but shows low thermal stability under harsh handling. Keeping that in mind, a process engineer might reach for bis(trifluoromethylsulfonyl)imide (NTf2−) instead, for more robust applications.
Evaluating Tradeoffs and Sustainability
Some worry about the environmental fallout from hexafluorophosphate, as it can release hazardous hydrogen fluoride on breakdown. This risk means researchers push for “greener” options. Choline-based ionic liquids, built from the same stuff in vitamins and biology labs, skip the dangers linked to fluorinated anions. Their simple structure (for example, choline chloride and urea, in what’s called “deep eutectic solvents”) makes them kinder for routine lab and industrial use.
Still, ionic liquids hold a place in clean energy tech, batteries, and metal separation. Their molecular structure—how the atoms and rings fit together and the charge balance—matters at every stage, from purity testing to process scale-up. It’s clear that the smart use of ionic liquids relies on looking past the catchy names and reading the formula and structure. This approach keeps industry and research grounded in sound chemistry, avoids waste, and encourages safer, more reliable products in daily life.
Taking a Closer Look at Ionic Liquids in Modern Labs
Electrochemistry lives at the edge of innovation these days, and some materials stand out for their fresh possibilities. One of them, 1-allyl-3-methylimidazolium tetrafluoroborate, often goes by its much shorter acronym, AMIM BF4. Chemists like me have watched ionic liquids burst onto the scene over the past decade. AMIM BF4 holds a special place on the bench thanks to a rare combination of stability, conductivity, and eco-friendliness.
What Makes AMIM BF4 Different?
Plenty of solvents run into trouble in electrochemical setups: water and standard organics bring limitations around volatility, reactivity, and corrosion. Ionic liquids like AMIM BF4 change the game because they hardly evaporate and refuse to ignite under normal conditions. The ionic nature gives solutions high charge mobility and non-flammability. These aren’t just abstract lab features—these properties open doors in battery research, capacitors, and devices where safety and staying power matter.
My early use of AMIM BF4 came during a project to improve electrodeposition for metal coatings. Traditional electrolytes reacted with air or broke down with slight temperature changes. AMIM BF4 ate up that challenge and kept the metal ions moving even after hours of current flow. Its wide electrochemical window let us push voltages higher without breaking the solvent, making thicker and smoother coatings possible. The hands-on difference proved more than theoretical—yield went up, waste dropped, and the equipment looked better at the end.
Where It Shines and Where It Struggles
Energy storage leads the charge for applications. Lithium-ion batteries grabbed headlines with ionic liquids because manufacturers want batteries that don’t burst into flames on your nightstand. AMIM BF4 stays stable with both lithium and sodium ions, and its low vapor pressure cuts leaks and fires. Supercapacitors also rely on AMIM BF4 for fast-moving ions and longevity over countless charge cycles.
Still, real-world obstacles stay in the picture. AMIM BF4 offers excellent ionic conductivity but trails some metallic salt solutions. Some sensitive electrodes react with the tetrafluoroborate anion, causing gradual degradation under heavy cycling. Corrosion, particularly on aluminum and steel, emerges without careful alloy choice or surface protection.
Balancing Performance, Cost, and Sustainability
Cost can sting early adopters. Synthesis of high-purity AMIM BF4 takes time, skill, and quality raw materials. Mass production brought the price down, but it still exceeds legacy organic solvents. Research groups drill deeper to recycle the solvent from cell to cell, and designs keep solvent exposures minimal. Down the line, wider recycling will drop costs and make use cases more viable outside of niche projects.
On the bright side, ionic liquids contain little to no volatile organic content. That matters for both workplace safety and environmental impact. Regulatory agencies pay attention to the waste streams from battery makers, and switching to AMIM BF4 from petroleum solvents means cleaner air and less toxic spills to clean up.
Steps for the Next Chapter
Lab experience shows AMIM BF4 can help solve problems that used to stump electrochemists for years. Achieving high energy density without sacrificing safety still draws sharp focus. Ongoing material tweaks—changing the anion or cation in the ionic liquid, coating reactive surfaces, or adding stabilizers—transform these ionic liquids from promising upstarts into proven workhorses.
Collaboration between chemists, device engineers, and manufacturers stands as the real accelerator. Each group brings insights on introducing AMIM BF4 in real-world devices, scaling up safe handling, and reducing waste. Knowledge-sharing has already knocked down barriers—and, based on my experience, the pace will keep picking up steam as industries catch on to what these new solvents can offer.