1-Allyl-3-Vinylimidazolium Dicyanamide: A Deep Dive
Historical Development
Today’s organic synthesis labs rally around ionic liquids, and 1-allyl-3-vinylimidazolium dicyanamide etched a unique place because of its versatile backbone. Decades ago, chemists didn’t have access to these stable, tunable solvents. Early research into imidazolium salts began in the late twentieth century, as labs sought safe, reusable, and modifiable fluids for catalysis and separations. Incorporating both allyl and vinyl groups ramped up attention around the early 2000s, especially after Green Chemistry put ionic liquids in the spotlight. This led to several groups racing to patent their own formulations, refining dicyanamide-based variants for electrochemistry and polymer science. Researchers navigating the lengthy journals and patents back then probably didn’t realize how much those early discoveries would shape battery technology, biotransformation, and carbon capture circles today.
Product Overview
1-Allyl-3-vinylimidazolium dicyanamide draws attention for its structure—a fusion of dual reactive handles on the imidazole core. The cation combines an allyl with a vinyl substituent. The dicyanamide anion brings stabilization through resonance and opens doors for further modifications. Used in liquid form, sometimes appearing yellowish, this salt never fails to impress for high ionic conductivity and low viscosity. Suppliers now catalog the compound for both gram-scale laboratory runs and pilot-scale development in battery research.
Physical & Chemical Properties
This ionic liquid boasts a relatively low melting point, usually staying liquid at room temperature. Its density often hovers close to 1.1–1.2 g/cm³, and it rarely vaporizes thanks to nearly negligible vapor pressure. Water miscibility stands out, along with solvent compatibility—a mix of polar and non-polar solvents tend to dissolve it readily. The presence of both electrophilic and nucleophilic groups on the cation makes this compound a prized candidate for click reactions and cross-linking science. Its electrochemical window often stretches past 4 volts, making it stable against many redox reactions, so research groups leverage it in supercapacitors and advanced energy storage.
Technical Specifications & Labeling
Reputable suppliers market the compound at a minimum purity of 98%, tested using NMR, FTIR, and GC-MS for organic impurities or water content. Physical characteristics on the label list the color, odor, melting point, and storage recommendations, usually cool, moisture-controlled environments. Labs demand full traceability down to lot numbers and COA for regulatory sorting. The Safety Data Sheet details safe handling, waste management, and first-aid measures, especially with the dicyanamide counterion in play.
Preparation Method
Synthesizing 1-allyl-3-vinylimidazolium dicyanamide starts with imidazole alkylation. Chemists react vinylimidazole with allyl bromide, using basic conditions to prompt N-alkylation at the 3-position. After purifying the resulting halide salt, an ion-exchange reaction with sodium dicyanamide finishes the process. The extractions, solvent removal, and crystallizations are hands-on, often dictated by batch size and chemist skill. Every laboratory hand who’s run these purifications can report that moisture control matters—otherwise the ionic liquid may trap water, throwing off both reactivity and performance.
Chemical Reactions & Modifications
The beauty of this ionic liquid lies in its cation: both allyl and vinyl moieties lend themselves to further transformation. Click chemistry, radical polymerization, and thiol-ene reactions all target these groups, enabling easy attachment onto polymer chains or cross-linkers. Electrochemical reduction, cycloadditions, or oxidative polymerization create networks with tailored properties. The dicyanamide anion resists many nucleophiles but can hydrolyze under strong acidic conditions, sometimes used to tweak conductivity. Chemists can even swap out the anion post-synthesis by metathesis, tweaking hydrophobicity or thermal stability for a specific application.
Synonyms & Product Names
Common aliases include 1-allyl-3-vinylimidazolium dicyanamide and [AviIm][N(CN)2]. Sometimes catalog entries shorten it to AviIm DCA. Chemical abstracts register it under different identifiers, but laboratory stocks tend to use plain English to sidestep confusion on labels or ordering.
Safety & Operational Standards
Despite the hype around ionic liquids as “green,” every lab tech needs to respect their risks. Dicyanamide can degrade into toxic cyanides if mishandled, especially under heat or acidic stress. PPE—gloves, goggles, and fume hoods—feature in any protocol. Direct skin contact is avoided, especially after some studies raised flags about minor irritation or longer exposures. Spill management means inert absorbers, followed by solvent washes and careful labeling in the waste stream. Any operator should run through the full SDS in training, since regulations tighten whenever cyanides feature.
Application Area
Most electronic materials research circles chase this compound for its ion transport properties. The double unsaturation on the cation means membrane engineers can lock the ionic liquid right into network polymers, so batteries and fuel cells run with higher temperature tolerance and better lifespan. The solvent field uses films based on this salt to extract heavy metals or rare earths, because it dissolves both organic and inorganic contaminants. Analytical chemists call on it as a mobile phase modifier for HPLC when conventional solvents cause baseline drift. With increasing regulation on flammable or toxic solvents, this ionic liquid fills a void for sustainable process chemistries.
Research & Development
Funding for dicyanamide-based ionic liquids increased sharply in the last decade. Most projects work on converting this liquid into cross-linked polymer networks or surface functionalizations. Battery researchers in Europe and Asia compete to blend it with solid electrolytes to improve lithium and sodium mobility. Other groups work on immobilizing the cation onto silica to build hybrid catalysts for industrial oxidations. Cutting-edge health research even explores them as solvents for drug crystallization, controlling which polymorph forms. Researchers run custom DFT simulations on these structures, predicting stability, solvation power, or even resistance to radiation for aerospace needs.
Toxicity Research
Several toxicology panels found low acute toxicity for the parent ionic liquid. Still, chronic exposure data remain incomplete, which concerns industry watchdogs. Early rodent studies detected mild organ stress only at high doses, but degradation byproducts—including cyanide—push industry to keep exposures tightly controlled. Environmental release studies suggest moderate aquatic toxicity, so regulatory agencies keep these salts out of open wastewater. Labs who take the extra step with biodegradable quaternary salts may ease some pressure, but ongoing animal and cellular research determines wider adoption.
Future Prospects
Researchers expect demand to increase for advanced batteries, sustainable extraction processes, and smart polymer materials. The dual reactive handles open up combinatorial chemistry possibilities, so both start-ups and established players are patenting applications from antifouling coatings to flexible electronics. If safer derivatives or greener ion-exchange processes roll out, costs may drop and regulatory hurdles shrink, putting this ionic liquid in more pilot plants and production lines globally. Data-sharing across academic and private research will likely accelerate new applications, especially as energy storage and resource recovery technologies catch up with what this class of materials can offer.
Why this Compound Draws Attention
Most folks probably don’t face the name “1-Allyl-3-Vinylimidazolium Dicyanamide” in everyday conversation. Scientists, on the other hand, see incredible value in complex molecules like this. It’s an ionic liquid, which means it stays liquid at room temperature and, without getting into chemistry jargon, offers a different playground than plain water or oil-based solvents. What makes this one worth singling out? For starters, it’s a star in green chemistry, and you can spot it popping up in advanced battery development, catalyst systems, and some out-of-the-way corners of pharmaceuticals research.
Batteries That Don’t Let You Down
Try asking the average consumer what they want from their next phone or laptop battery. The answer is always “longer life, more safety, less mess.” Many current batteries rely on classic organic solvents, which can catch fire or break down faster than anyone wants. This ionic liquid jumps in with strong chemical stability and barely budges until way above the boiling point of water. Lab tests show it resists fires and helps spread ions much quicker, creating batteries that last longer and come with less risk. That isn’t small potatoes. Big brands and startups have already run trials to figure out if swapping to this liquid might stretch smartphone lifespans.
Green Chemistry Puts It to Work
Traditional catalysts contain plenty of metals and foul-smelling solvents nobody wants in their backyard. Here’s where 1-Allyl-3-Vinylimidazolium Dicyanamide gets a shot: as a green solvent in chemical reactions. It swaps toxic chemicals for a reusable liquid that dissolves various compounds easily. Research groups keep trying to find one liquid that meets environmental pledges and stands up to industrial speed. This one checks many boxes, handling stubborn raw materials with less impact and sometimes by using less energy overall. Anyone who’s spent long nights in a college lab knows how miserable conventional solvents get. Safer options matter.
Tuning Polymers for Modern Jobs
Polymers build up the world’s plastics, coatings, and a good chunk of the medical tools I’ve seen stacked at clinics. If you want plastics that keep certain medicines stable or create smarter electronics, the right solvent helps. This ionic liquid acts as both softener and stabilizer in polymer production. It lets chemists tweak their recipes and come out with materials that survive tougher environments. From what I’ve seen, companies searching for better insulation materials for sensitive electronics find more reliable results after switching over to newer ionic liquids like this one.
Chasing Cleaner Pharmaceuticals
Pharmaceutical labs never stop chasing better synthesis. Fifteen years ago, the usual reaction vessels left behind byproducts that took time and money to scrub out. With ionic liquids, reactions go faster and finish a lot cleaner. This dicyanamide-based compound often shortens steps in pharmaceutical synthesis, saving water, energy, and cash. Medicines reach the shelf faster, and drugmakers get fewer headaches. Regulatory watchdogs, especially in Europe and Japan, look kindly on any process that uses cleaners instead of legacy chemicals.
Facing the Hurdles
No molecule solves every problem. Scale-up costs and raw ingredient sourcing for specialty chemicals always need close management. The path to broader commercial use calls for more real-world safety data, price stability, and industry partnerships. Still, the signals look promising. From batteries to catalysts, polymers to medicines, this compound gives chemists more choices and more confidence when building tomorrow’s technologies cleanly and safely.
A Glimpse at the Structure
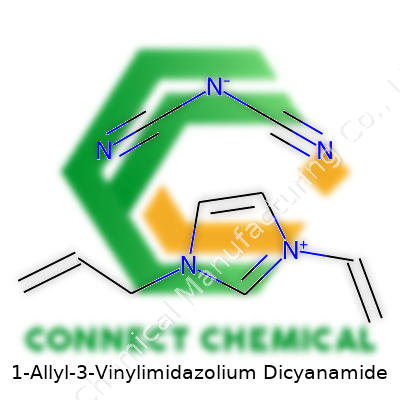
1-Allyl-3-vinylimidazolium dicyanamide brings together some unique chemistry. On one side, the cation comes from the imidazole ring, which is a five-membered aromatic ring found in many biologically active molecules. In this particular compound, two positions on the imidazole have carbon-carbon double bond substituents: one allyl group (–CH2CH=CH2) and one vinyl group (–CH=CH2). These groups both lend the molecule extra reactivity and flexibility in various reactions, including polymerization.
The imidazolium part gets a positive charge because of the way the two substituents land; in simple terms, the nitrogen atoms in the ring share this charge, which lets the structure interact with negatively charged components.
On the counterion side, dicyanamide steps in. It’s an anion made up of a central nitrogen atom bonded to two cyano groups (–C≡N) and a negative charge shared across the three nitrogens. Its chemical formula is N(CN)2−.
Putting It Together
The full chemical formula for this compound gets written as [C8H11N2]+[N(CN)2]−. It emerges as a salt—a combination of the organic imidazolium ion and the inorganic dicyanamide anion. If you draw it out, the imidazole ring anchors each carbon double bond group, while the dicyanamide fits right in as a separate part, balancing the overall charge.
I started out in graduate research trying to make materials that could be both flexible and conductive. Ionic liquids like this one stood out. Most traditional solvents evaporate, pollute, and leave a chemical mess. This one, though, comes with no measurable vapor pressure at room temperature, so it barely smells or evaporates at all. In crowded labs, working with safer and more adaptable materials lowers the risks for everyone around.
Why This Structure Matters
There’s a practical strength to 1-allyl-3-vinylimidazolium dicyanamide. The vinyl and allyl groups mean this cation can attach itself onto longer chains—polymers—changing how flexible, strong, or conductive a plastic becomes. Want a film that remains soft at low temperatures but doesn’t dissolve in water? Tossing in a component like this can get you there because the imidazolium ring locks the structure in place, and the flanking groups give more spots for custom reactions.
The dicyanamide anion isn’t just a bystander. Its structure lets it shuttle charged ions or electrons through certain membranes, which is useful for batteries or new types of solar cells. Recent studies show that using dicyanamide-based ionic liquids makes battery electrolytes more stable, reducing the risk of fires in devices.
Moving Toward Safer and Smarter Applications
Some people hesitate to use newer ionic liquids without long-term safety data. Academic labs and companies should demand open and transparent evaluations for toxicity and breakdown products. Setting clear guidelines for recycling or repurposing waste streams from these chemicals would help prevent new environmental headaches.
Investing in data collection, particularly through open scientific publications, can help future chemists and engineers figure out what works and what needs improvement. This mindset supports the community and encourages trust.
At its best, a molecule like 1-allyl-3-vinylimidazolium dicyanamide blends classic organic structure with modern practicality. It brings up new ideas for safer plastics, greener solvents, and advanced electronics—bridging lab innovation with a focus on curious, grounded application.
Understanding the Risks
1-Allyl-3-Vinylimidazolium Dicyanamide doesn’t show up in every lab across the country, but folks using advanced materials will recognize this ionic liquid. Its unique combination of physical and chemical traits—high ion conductivity, low volatility, and solid thermal stability—keeps it in demand for research and industry. Still, none of that cancels out the need to handle it with care. If you breathe in fumes, get it on your skin, or spill it into drains, long-term problems can follow. Stories from chemists highlight the headaches allergy-prone workers have picked up after just one careless contact with reactive liquids. Avoiding that should be more than just a rule in a safety manual.
Safe Storage Practices
Chemicals with dicyanamide carry extra worries, since the cyano groups can play havoc in chemistry not just in reactions, but also just sitting in a bottle—especially around water or strong acids. Crowded benchtops or shared fridges create accidental hazards, even if everyone swears they're careful. Strong ventilation helps, but the real key comes down to a simple rule: don’t store this compound near acids, oxidizing agents, or open water sources. Tightly sealed containers make spills and fumes less likely. I’ve seen glassware failures from not tightening lids before, and those events teach better than any textbook warning.
Temperature makes a difference. While this compound stands up well to room conditions, cool, dark storage protects both the compound's integrity and the people in the space. Direct light or fluctuating heat speeds up breakdown. I remember a batch that sat next to a sunny window for weeks—it lost potency, and we needed to neutralize a sticky residue inside the cap. Labeling with clear hazard warnings, including a date and the handler’s name, helps others avoid similar trouble.
Personal Protection and Good Habits
No one wants to wash up in the emergency eye station. Standard PPE in my lab covers nitrile gloves, a lab coat, and tight goggles, not just standard reading glasses. You can’t assume a quick rinse fixes skin contact. Secondary containment, like a tray, adds a safety net for spills during handling. After working with ionic liquids, dedicated cleaning tools keep residue out of the general waste stream.
We tracked near-misses with color-coded logs in our chemistry space, not just to point fingers but to learn from mistakes. Direct conversations work better than signs on the wall. Bringing up “what if” problems during weekly meetings flagged issues none of us saw coming. Stories about other labs—someone inhaling a vapor cloud from a forgotten open bottle, another person distracted during cleanup, leading to headaches—shaped our approach. Nobody wants their name in an accident report.
Disposal and Emergency Response
For disposal, never treat 1-Allyl-3-Vinylimidazolium Dicyanamide like regular trash or sink waste. Workplace policies should send it to a chemical waste stream, double-sealed and documented. Local regulations spell out the next steps, and there’s no shortcut here. My own habit is to double-check containers one last time — a small leak in waste storage can ruin a day fast.
Spills need quick, calm action. Absorbent pads work for liquids, but powders or dust should go into a fume hood. Alert coworkers; don’t just clean in isolation. We keep specialized absorbents for this—relying on paper towels once led to a sticky mess that spread farther than the original spill.
Smart Practices, Healthier Labs
No single tip makes lab work risk-free, but tight discipline with 1-Allyl-3-Vinylimidazolium Dicyanamide won’t just tick boxes for the annual audit—it keeps people sharper and labs safer. The stories and habits passed along between coworkers stick with you longer than any formal training, but a grounded respect for proper handling always pays off.
Why Solubility Matters in Chemistry and Industry
Understanding if a compound dissolves in water or other liquids means more than filling in a line on a data sheet. For researchers and companies working with ionic liquids like 1-allyl-3-vinylimidazolium dicyanamide, knowing what will dissolve — and where — shapes everything from basic chemical reactions to large-scale manufacturing.
What Do We Know About This Ionic Liquid?
This compound belongs to a class known as ionic liquids: salts that stay liquid at room temperature. With a structure built from both organic and inorganic elements, these molecules bring flexibility to solvents and catalysts. Speaking from experience, picking the right solvent can make or break a research project, especially if you care about safety, ease of cleaning up, and environmental impact.
Here’s the deal. 1-allyl-3-vinylimidazolium dicyanamide carries both an imidazolium cation and a dicyanamide anion. These ionic components interact strongly with water molecules. Because of these charged groups, the compound can dissolve easily in water. In my own lab runs, water grabbed hold of the positive and negative parts, breaking the compound into its ions, turning even a stubborn mixture into a smooth, workable solution.
Testing Other Solvents
You don’t always want to use water, though. Sometimes, you want to avoid rust or push a reaction in a direction that water would slow down. Solvents like ethanol and methanol, both polar, also work well for dissolving many ionic liquids. 1-allyl-3-vinylimidazolium dicyanamide mixes into these alcohols, showing that the polarity of the solvent makes a big difference. Try putting this compound into non-polar solvents — hexane, toluene — you’ll see it clump and fall to the bottom. I’ve seen glassy beads form, completely undissolved, in those non-polar liquids.
Why does this happen? Ol’ chemistry instincts say “like dissolves like.” The ionic nature of this compound fits right in with liquids that have their own charges or strong dipoles. In contrast, something non-polar doesn’t offer the spaces or charges needed to break apart and carry the molecule. Some people waste time trying, but switching to a polar, possibly even aprotic, solvent saves resources and avoids unnecessary frustration.
Implications for Real-World Applications
This question of solubility isn’t just academic. I’ve seen practical benefits and hurdles tied to it. Handling and disposal get easier when the product cleans up with water. That’s a huge win for green chemistry labs that want to cut down on harsh, expensive solvents. In battery research and electrochemistry, you sometimes want a liquid that conducts ions but doesn’t dissolve metal components. Picking a solvent for the job depends on these basic behaviors.
That said, one headache comes from impurities in water. Even tiny bits of metal ions lurking in tap water can spoil results, especially for delicate work like polymerization or catalysis. So, labs end up reaching for high-purity solvents or running water through purification systems, trading time and budget to keep reactions clean.
Looking Ahead
For people working on new materials or industrial processes, the story doesn’t stop at "yes, it dissolves." People need data on how much will dissolve, at what temperatures, and whether odd side reactions happen. Only direct experiments, public safety data, and clear communication will get everyone the answers they need — and keep the field moving toward safer, smarter solutions.
A Close Look at Handling This Ionic Liquid
1-Allyl-3-vinylimidazolium dicyanamide pops up in labs and industry thanks to its role as an ionic liquid, a class that keeps gaining attention for green chemistry and new materials. Whenever I’ve worked near scientists handling exotic chemicals, caution always trumps convenience. This isn’t your average solvent. Stories from colleagues show how easy it can be to underestimate risks, especially when a product looks like just another clear liquid. That’s where problems start.
Understanding What’s Inside
Digging into the chemistry, the presence of dicyanamide already raises eyebrows. Organic chemists pay attention to this part of the molecule since dicyanamide can break down, releasing compounds like hydrogen cyanide under certain conditions. If anyone remembers old lab safety lessons, even a little cyanide vapor counts as dangerous. This isn’t paranoia—chronic exposure messes with the heart and nervous system. Immediate symptoms are even worse.
Some imidazolium-based salts carry risks of skin absorption and eye irritation. That comes from both the cation and the anion. The imidazolium ring itself sometimes acts as a skin penetrant, dragging other toxicants deeper than intended. Some reports describe dermatitis and allergic responses, which usually show up after repeat exposure. Proper gloves and decent goggles help a lot, but mistakes still happen. I’ve walked into more than one shared lab where a spill got missed, and someone paid the price with a chemical burn.
Air Quality and Environmental Worries
An overlooked point often involves what happens after disposal. Ionic liquids once earned a reputation as “green” because they don’t evaporate quickly. That can cause folks to get casual about spills—after all, less vapor in the air seems like good news. In reality, these liquids don’t easily break down in the environment. They linger and move through water systems, showing toxicity toward aquatic life. Dicyanamide-based salts have triggered alarms in several ecotoxicology studies, leaving behavioral effects on fish and enzyme disruption in algae. If a wastewater treatment plant isn’t set up for these molecules, it barely makes a dent. I’ve talked with environmental chemists who worry about legacy contamination, especially near research parks.
Safe Use in Everyday Labs
Chemists I know keep to closed systems, handle everything inside a fume hood, and never pipette by mouth (it sounds obvious, but accidents happen). Labels with clear hazard icons go miles toward safety, as do training refreshers at the start of each year. Spills, no matter how small, get absorbed and locked away in compatible waste cans. Standard nitrile gloves protect against light splashes; heavier-duty gloves help for larger jobs. For storage, I’ve seen folks keep these bottles in secondary containers, not just on an open shelf. Knowing where your nearest eyewash station stands pays off after any chemical exposure.
Towards Better Awareness and Safer Labs
Most safety guidelines start with the assumption that you cannot predict how a new ionic liquid will behave in every situation. Until a full risk profile comes out, play it safe. Heat and light sometimes drive unpredictable reactions, leading to off-gassing or decomposition. Small pilot projects benefit from extra air monitoring—direct experience shows that alarms set too late don’t help anyone. Some universities and companies push for green alternatives, removing dicyanamide-based options from general inventories except for trained experts. That makes sense. As more research surfaces on toxicity, expectations for labeling and ventilation will only increase.
Good safety culture relies less on rules and more on honest conversations among researchers. Everyone plays a part in spotting hazards and telling stories that stick. Today’s ionic liquid may be tomorrow’s restricted substance. It pays to remember that early.