1-Aminoethyl-3-Methylimidazolium Bromide: A Deep Dive
Historical Development
Curiosity has always driven chemists to search for new compounds that push boundaries in research and practical uses. The imidazolium ionic liquid family started as an answer to high volatility issues found in older solvents. Early work in the late 20th century built the foundation for room-temperature ionic liquids, and with it came possibilities for greener chemistry. Researchers tinkered in labs, modifying basic imidazolium cores, adding various functional groups. Adding an aminoethyl group created 1-Aminoethyl-3-Methylimidazolium Bromide, which brought new life to synthesis and extraction processes, especially where classic solvents fell short on selectivity or environmental friendliness. Years of effort and trial-and-error gave birth to a more user-friendly, tunable ionic liquid that now anchors several laboratory processes.
Product Overview
1-Aminoethyl-3-Methylimidazolium Bromide stands out for its tailored blend of reactivity and structural stability. It typically appears as a solid or viscous liquid, depending on storage temperature. The cation, armed with an aminoethyl group, cradles the methylimidazolium ring, matched with a bromide anion. This pairing opens up a set of chemical functionalities not found in bulkier, less nimble salts. Researchers pick this compound for versatility; engineers look at it for potential in extractions and catalysis, and pharmaceutical scientists eye its unique solvation properties.
Physical & Chemical Properties
Most samples carry a pale yellow to off-white color. The melting point hovers slightly above room temperature, making storage conditions important to maintain workability. As for solubility, it dissolves readily in water and certain polar organics, letting users tap into creative mixtures. It sports good thermal stability for day-to-day lab use. The density registers between 1.1 to 1.3 g/cm³, and the ionic character supports low vapor pressure. Chemically, the amino group allows interesting nucleophilic behavior, opening doors to modification routes and selective interactions. The ionic nature shields the compound from rapid hydrolysis under ambient conditions, though stronger acids or bases will still break it down.
Technical Specifications & Labeling
Manufacturers usually label the product with CAS No. 537650-99-6. Purity grades commonly reach above 95%, with confirmation via NMR and elemental analysis. Moisture content appears on most labels, due to the hydrophilic amino group. Handling guidelines print clearly on the packaging, warning of potential eye and skin irritation. Packing comes in sealed glass or HDPE bottles with a desiccant pouch to avoid caking and degradation. Checklist for users: always look for year of manufacture, batch number, storage conditions, and hazard pictograms before use in sensitive protocols.
Preparation Method
Classic routes use direct alkylation reactions. Lab-scale synthesis usually starts with 1-methylimidazole, which reacts with bromoethanamine under controlled heating. The reaction vessel, often a round-bottom flask equipped with a stir bar and reflux condenser, provides the perfect environment for the base to capture the bromide and leave the desired product. Some opt for microwave-assisted methods, which speed the reaction and reduce byproduct formation. After the main reaction finishes, the mixture goes through careful solvent washes and, in some cases, column chromatography. Residual organic material gets removed under reduced pressure, and crystals form on cooling or through antisolvent-induced precipitation.
Chemical Reactions & Modifications
The primary amine on the ethyl arm gives a direct handle for further chemical transformations. Researchers often use acylation or reductive amination to diversify possible derivatives on the imidazolium platform, extending application into new territories. The methyl position on the imidazole ring also provides opportunities for functionalization. Bromide can swap for other anions, tuning the ionic liquid’s physiochemical traits for specific solvents or environments. Several labs across Europe and Asia report success using this compound as a phase-transfer catalyst, leveraging the charged, lipophilic nature to shuffle small molecules across interfaces in multi-phase systems. The aminoethyl group enables covalent anchoring to surfaces or polymers, facilitating use in supported catalysts or polymer electrolytes.
Synonyms & Product Names
Beyond its main name, chemists may spot it under 1-(2-Aminoethyl)-3-methylimidazolium bromide, N-[2-(1-methylimidazolium-3-yl)ethyl]amine bromide, or as its short-hand abbreviations like [AEMIM]Br in research articles. Catalogs from chemical suppliers may add their own stock codes or registry numbers, but all roads lead back to the same core structure. Old literature sometimes uses different notations, so cross-checking structures becomes essential for anyone doing a deep dive through publications.
Safety & Operational Standards
Safety never takes a day off in the lab. While 1-Aminoethyl-3-Methylimidazolium Bromide brings lower volatility compared to typical solvents, direct exposure may irritate eyes and skin. Always pull on gloves and goggles. Never pipette by mouth; accidental oral exposure causes headaches, drowsiness, or worse, especially at higher concentrations. Spill cleanup proves straightforward thanks to water solubility, but avoid letting material get down the drain unless permitted by local waste handling rules. Work in ventilated spaces, and keep a spill kit nearby. Storage in tightly sealed containers away from heat and moisture preserves shelf life and prevents accidental hydration, which could alter handling or reactivity. Some suppliers include a safety data sheet (SDS) with every order to summarize hazard pictograms and first aid advice.
Application Area
Industry and research both lean on this ionic liquid for its selectivity and operating stability. In organic synthesis, the compound acts as a medium where traditional solvents fail—especially in reactions sensitive to air or high temperatures. Separation science benefits when users deploy it for ion-exchange or liquid-liquid extraction tasks. Some studies report efficient use in CO₂ capture or catalysis, reducing both toxicity and environmental footprint compared to legacy solvents. As an electrolyte additive, battery makers see gains in conductivity and cycle stability. Medicinal chemists experiment with the compound in drug extraction, where purity and yield often determine success or failure. Polymer scientists use the aminoethyl arm for grafting, tailoring new macromolecules for smarter membranes.
Research & Development
Innovation thrives in every corner the compound touches. Many research teams keep expanding uses, from greener reaction routes to analytical chemistry. Interest in task-specific ionic liquids keeps rising, and 1-Aminoethyl-3-Methylimidazolium Bromide finds itself at the center. Recent work pushes applications in the direction of biocatalysis, pairing the compound with enzymes for product synthesis under mild conditions. Optical sensor development uses the compound as a matrix for immobilizing chromophores or enzymes. Materials science circles see promise in blending with nanoparticles to create soft matter with tunable magnetic, thermal, or mechanical properties. New protocols in environmental science show the compound aiding recovery of precious metals from e-waste and low-grade ores.
Toxicity Research
Data from acute and chronic exposure studies highlight a relatively low toxicity profile, especially compared to many legacy solvents and salts. Still, questions remain about longer-term bioaccumulation in aquatic systems. Early toxicology reports show minor skin and eye irritation at standard lab concentrations, but sustained or high exposure can push effects into headache, dizziness, or mild neurotoxicity. Studies with model organisms such as Daphnia or zebrafish suggest moderate inhibition of growth and reproduction at higher levels. Biodegradation remains slow in plain water, so waste handling protocols err on the side of caution. Ongoing research investigates breakdown pathways and remediation approaches, including tailored biodegraders and advanced oxidation processes to manage spent liquid stock.
Future Prospects
The road ahead for 1-Aminoethyl-3-Methylimidazolium Bromide looks promising, bolstered by mounting demand for smart solvents and functionalized reagents. Market forces nudge manufacturers to scale up output and reduce costs, which will push the compound further into industrial workflows. Scientists talk about new phases of ionic liquid research, eyeing tighter connections between function and structure. More mainstream use in green chemistry lies just over the horizon, promising lower emissions and cleaner production streams. Every year, applications in electrochemistry, pharmaceuticals, and waste upcycling broaden as understanding deepens. Breakthroughs in toxicity mitigation and faster, efficient syntheses could cement this ionic liquid as a standard tool for a cleaner, more innovative chemical industry.
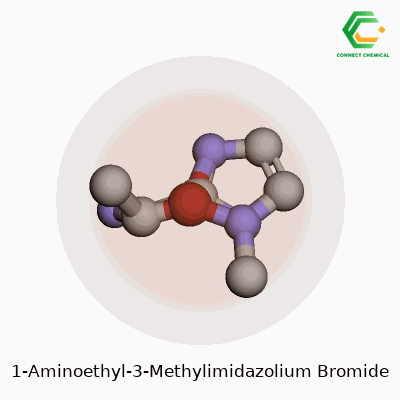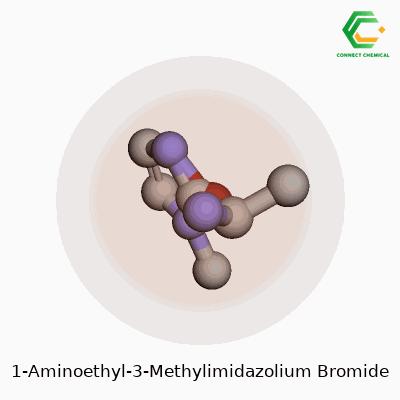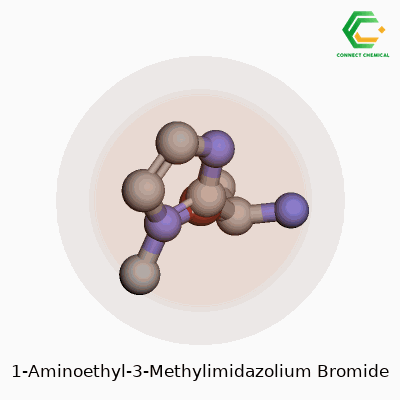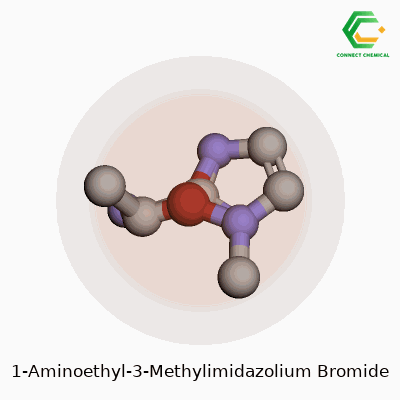
Chemical Structure: Breaking It Down
Chemistry becomes clearer when you see a picture, so let’s put the key details into words. 1-Aminoethyl-3-methylimidazolium bromide belongs to the family of ionic liquids. Its backbone rests on the imidazolium ring, a five-membered structure with two nitrogens. Now, swap the 1-position hydrogen with an aminoethyl group – that’s a two-carbon chain capped by an NH2. Toss a methyl group onto the nitrogen at the third spot. The molecule as a whole teams up with a bromide anion for electrical neutrality.
People often use chemical formulas to keep things short and sweet. For this compound, the cation is written as C6H12N3+, matched with a single Br- anion. The benzene-like imidazole core keeps everything planar, but the bulky side groups change how this material flows and dissolves.
Why Chemists Care About This Structure
Getting the structure down matters because it tells you what this material can actually do. The aminoethyl group adds stickiness: It forms hydrogen bonds, grabs hold of polar molecules, and attracts metal ions. Lab researchers see value in these features – for example, purifying rare earth metals or yanking organic pollutants out of water.
Imidazolium compounds like this one are well-known for forming stable ionic liquids. These don’t vaporize or burn easily, and they often work as green solvents. People have asked for solvents that don’t stink, catch fire, or harm workers. So, companies and universities keep chasing new ionic liquids for safer chemistry or lower-energy processing.
Why the Details Make a Difference
Over years working in synthetic labs, I’ve noticed a recurring theme: Small tweaks in side groups lead to big shifts in behavior. With 1-aminoethyl-3-methylimidazolium bromide, the extra amino group means better mixing with water and easier bonding with carbon dioxide or transition metals. Traditional imidazolium salts without the amino group act hydrophobic, stubborn in water and more locked into organic phases.
The bromide counterion also brings a specific twist. It’s large, not very polarizing, and relatively safe compared to heavier halides like iodide. Swapping out bromide for a more toxic partner shifts the whole risk profile, so sticking with bromide has made the compound more attractive for testing clean-up applications and as part of solid-state batteries.
Challenges and Practical Considerations
The real-world story isn’t all rosy. Imidazolium bromides sometimes absorb water from the air, so powder starts clumping unless you seal them up tight. Bromide itself can trigger corrosion, especially in metal tank linings, so storage containers matter. Some researchers report minor toxicity to aquatic life, especially if these liquids pile up outside lab walls.
A closer look at scale-up shows wrinkles too. Cost remains high because raw materials involve several synthetic steps, and the need for high purity stretches production times. Cleaner routes, like direct alkylation or swapping in greener solvents, could trim costs and hazards.
Looking Forward: Better Chemistry by Design
There’s plenty of room to make these compounds safer, cheaper, and easier to recycle. Much of the promise for 1-aminoethyl-3-methylimidazolium bromide circles back to thoughtful changes in its chemical structure: swapping side chains, changing the anion, or designing reusable syntheses. Chemists keep pushing for models that predict how structure shapes function – not just for patents, but to raise the bar for worker safety, environmental impact, and overall usability.
Why Chemists Value This Ionic Liquid
1-Aminoethyl-3-methylimidazolium bromide might not catch anyone’s eye in a pharmacy or on a hardware store shelf, but people in labs recognize its wide utility. This ionic liquid belongs to a class of chemical salts that stay liquid at room temperature. The scientific community caught on to their usefulness once someone realized they dissolve all sorts of tricky compounds that water, alcohol, or acetone just can’t touch. The presence of the aminoethyl group opens up even more options—especially where selectivity matters.
Advancing Green Chemistry
Organic solvents still dominate industry. Those come with problems: toxicity, flammability, environmental hazards. 1-Aminoethyl-3-methylimidazolium bromide offers a greener alternative. Its low volatility means you aren’t going to be breathing harmful vapors. Researchers use it to swap out classic solvents in processes such as cellulose dissolution. Since breaking down cellulose leads to biodegradable plastic production and sustainable textiles, this compound plays a part in moving these sectors closer to reduced waste and carbon footprints.
Catalysis and Reaction Media
Catalysis gets a huge boost from ionic liquids. In routine lab work, this compound serves as both a solvent and a stabilizer for transition metal catalysts. Suzuki and Heck couplings—important methods for putting carbon atoms together—run smoother and with higher yields in this medium versus traditional setups. Not needing extra stabilizers cuts down on steps and reduces byproducts. In such settings, I’ve noticed less hassle tracking down impurities post-reaction. Better purity means results you can trust, especially in pharmaceutical research.
Electrochemistry and Energy Storage
As batteries and capacitors grow more advanced, finding electrolytes that perform well and stand up to regular use stays key. This bromide salt catches attention for its wide electrochemical window. That means batteries using it can operate at higher voltages, storing more energy in less space. These features put it on the shortlist for next-gen lithium-ion batteries. In the field, a stable electrolyte can mean the difference between a battery that fizzles on a cold morning and one that holds up through real-world abuse.
Materials Science and Synthesis
Material chemists lean into this ionic liquid for its role as a template. During the synthesis of nanoparticles or polymers, it helps control size and shape at the microscopic level. With more uniform particle formation, coatings, sensors, and targeted drug delivery systems perform better. In my own work, I remember days when achieving tight particle size distribution seemed impossible with traditional solvents—switching to an ionic liquid like this saw problems evaporate overnight.
Problem Solving in Research & Industry
Because 1-aminoethyl-3-methylimidazolium bromide supports reactions under milder conditions, labs save energy and time. Lower reaction temperatures cut down on costly cooling and cut risks of thermal decomposition, improving safety. As more industries seek to "green" their supply chains, they turn to ionic liquids for both production and post-processing steps—recovering valuable catalysts or purifying products without a heap of waste solvents.
What Lies Ahead
Stepping up to cleaner production means more than dumping new compounds into old processes. With more regulatory attention on solvent emissions and chemical safety, materials like 1-aminoethyl-3-methylimidazolium bromide give a pathway toward meeting stricter standards. Collaboration between academia and industry could unlock more ways to use less hazardous materials—making life easier for chemists and safer for everyone else.
Everyday Encounters with Product Purity
You open a box of flour, pour it into a bowl, and notice a small clump or a dark speck. That pause before deciding if it’s still safe isn’t unique to home kitchens. Across industries, whether turning out medicine or food, that split-second judgment traces back to the same idea—purity tells a product’s story long before it reaches anyone.
Why Purity Affects More Than Just Labs
I remember working in a small family bakery. It was easy to spot the difference between fresh, high-quality ingredients and ones that had been sitting a bit too long. A batch of sugar with off-color grains subtly shifted the flavor of every cake. People sense these changes. Now take that awareness to the next level: with medications, purity doesn’t just mean better taste or texture—it shields us from harm.
A cosmetic cream, for example, isn’t just a jar with an ingredient list. If impurities linger—things like trace metals or leftover solvents—the skin can flare up or, over time, experience much worse. Companies aren’t just showing off when they claim 99.9% purity; they’re protecting trust and health. Chemically, small impurities in a final product can sometimes spark powerful allergic reactions or even cause medications to become less effective.
Physical Appearance Sends Signals
Before any lab test, people judge with their eyes. Dull, inconsistent powder, off-color tablets, oily films on top of liquids—all point to something off. These aren’t just visual details; they can be warning signs about poor mixing, contamination, or even counterfeit material. My own experience in quality assurance taught me this well: running my fingers through grain samples, feeling for clumps or fine dust, I understood how physical appearance connects to safety on a gut level.
Pharmaceutical and food companies screen products visually before any advanced testing starts. This practice saves lives and protects businesses from disastrous recalls. A team that misses the appearance of cloudiness in a supposedly clear liquid might miss the start of bacterial growth or a chemical reaction that could cause harm.
Moving Beyond the Surface
In many settings, measuring purity needs solid lab equipment—chromatography columns, titrators, and the expert eyes of trained analysts. Still, physical appearance remains the front line, functioning as a fast filter. A clear, crystalline solid, bright white powder, or liquid without sediment tells buyers and users that someone cared about the process from the start. Fail to pass these eye tests, and you can bet no one’s in a hurry to check deeper.
Solutions exist, but they demand constant vigilance. Regular training in spotting appearance changes, along with investment in simple but effective tests, helps small operations just as much as mega-factories. Collaboration between suppliers, manufacturers, and inspectors makes it harder for unsafe or subpar products to slip through. For shoppers or patients, reading appearance cues and purchasing from trusted sources turns into a habit, not a chore.
Pushing for Better Practices
Purity, backed by a healthy respect for how things look and feel, sets the bar for every product’s journey. Whether mixing batter at home or overseeing a pharmaceutical line, the principle stays the same: what we see and test shapes everything that follows. In an age where quick fixes tempt everyone, sticking with careful, practiced scrutiny matters more than ever.
Understanding the Material’s Personality
If you’ve ever worked in a lab, you know some chemicals need a little more attention than others. 1-Aminoethyl-3-methylimidazolium bromide fits right into that category. It’s an ionic liquid, with plenty of promise in fields ranging from catalysis to energy storage. It’s not just slippery science—this stuff really moves into everyday workspaces where people need to rely on safety and consistency.
Those Labels Don’t Lie
You crack open a fresh bottle and spot those big bold warning symbols. It’s a reminder—this compound deserves respect. Direct skin contact irritates, and if those tiny crystals find their way into eyes, trouble comes fast. I once watched a distracted tech rub his eye after handling an ionic liquid. He missed a crucial rinse and spent the next hour blinking and cursing at the eyewash station. Good gloves, tight goggles—there’s no room for sloppiness. Always use clean nitrile gloves, and toss them out as soon as you’re done. Don’t wait for the stuff to eat through the material.
Fume hoods aren’t optional. Out in the open, you take too much risk breathing vapors or scattering fine dust. So, steady hands under the hood create a barrier between you and invisible dangers.
Keep It Cool, Keep It Dry
The stories about someone storing a sensitive chemical on a shelf near a hot steam pipe don't end well. 1-Aminoethyl-3-methylimidazolium bromide stays stable under the right conditions but, throw temperature swings or moisture into the mix, and you’re rolling dice. Damp air and warmth accelerate breakdown.
Set storage at cool room temperature, away from light and heat—no short cuts. Desiccators work well for keeping humidity low. Sealing the container tightly after every scoop makes contamination less likely, and prevents the compound from caking or reacting with air. As a rule, containers live on shelves dedicated to chemicals that play well together; acids nearby only create future headaches.
Spill Response—No Surprises
Eventually, someone in the lab drops something. Spilled powder spreads fast, especially if people walk through it without noticing. Training matters. Staff learn to contain and scoop up dry spills using gentle sweeping and damp cloth, not a vacuum or compressed air that throws particles into the air. For liquid messes, absorbent pads and a dash of patience prevent wider contamination.
Waste disposal needs forethought, too. No one slips the stuff down the drain. Strict guidelines steer waste into labeled hazardous containers, so nobody downstream faces risks they didn’t sign up for.
Safer Spaces for Everyone
I’ve seen strong safety cultures build trust across teams. Senior researchers show juniors how to treat tricky chemicals the right way, so habits stick. Monthly safety drills, posted reminders, and keeping protective gear in easy reach create an environment where mistakes get caught before causing serious problems.
Newcomers often want to rush. Slowing down, walking through every step of storage and handling, and never skipping the safety data sheet save pain later. Better to repeat the safety talk than teach the hard way by accident. Good habits lead to fewer incidents, and a lab that keeps humming along.
Understanding What’s at Stake
In the world of ionic liquids, 1-Aminoethyl-3-Methylimidazolium Bromide crops up now and then, promising niche applications in chemistry, extraction, and separation processes. Researchers keep experimenting with these types of chemicals, eager to discover something better, greener, safer. My own stints in academic labs taught me to check the safety data before anything else—especially if the substance has an imidazolium ring, which often brings unpredictability to the table.
Scratching for Safety Data
Trying to dig up straightforward answers about the safety of 1-Aminoethyl-3-Methylimidazolium Bromide turns into a wild goose chase. Major databases frequently skip detailed information for this specific compound. The handful of published academic articles hardly mention safety at all, staying laser-focused on its performance in experimental setups. It’s frustrating, having to piece things together from bits of related compounds and general imidazolium safety profiles.
The European Chemicals Agency database doesn’t list it. The U.S. National Library of Medicine glosses over any deep toxicity analysis. Chemists in industry might run across it but must rely on Material Safety Data Sheets pulled from manufacturers, often filled with generic warnings, not hard numbers. This lack of specifics leaves a gap. It’s tough to make adequate decisions about chemical storage, disposal, and handling with such spotty information.
Looking at What’s Known About Similar Chemicals
In academic environments, there’s a rule of thumb: treat every unfamiliar ionic liquid as potentially hazardous until proven otherwise. Many imidazolium-based salts pose hazards. Some irritate skin and eyes; others harm aquatic life. Their toxicity usually comes down to the substituents on the imidazolium ring and the anion paired with them.
A lot of research centers around the more common 1-butyl-3-methylimidazolium salts, and these frequently rank as mild to moderate toxicants for aquatic species. Human data is scarce, but what little information exists points to eye and skin irritation. I’ve seen how colleagues working with similar compounds turn to gloves, goggles, and fume hoods, refusing to gamble with poorly understood chemicals.
Risk Management Is Not Guesswork
The gaps in public safety and toxicity information signal a deeper flaw in the way some novel chemicals enter labs and, eventually, industry. Regulatory agencies like the EPA and ECHA demand safety assessments, but new substances get a pass until they become widely used or spark environmental concern. I remember the scramble to fill out SDS forms for new chemicals in my own research, often cross-referencing closely related compounds and relying on standard PPE as a safety blanket.
Practical Steps to Fill the Safety Void
The only way forward lies in building a bigger, public database of experimental toxicology data for less common ionic liquids like this one. Journals and academic conferences ought to highlight not just effectiveness in new processes but also include full safety profiles on novel chemicals tested. Funding agencies could attach requirements: share safety data with every grant-supported publication. With researchers, manufacturers, and regulators onboard, future teams would avoid the patchwork approach that dogs today’s users.
Until that system exists, the best practice comes from sticking hard to safety. Wear protective gear, use fume hoods, avoid direct contact, and dispose of waste according to the strictest applicable regulations. Taking shortcuts invites real risk, especially with chemicals whose impacts still hide somewhere in the scientific unknowns.