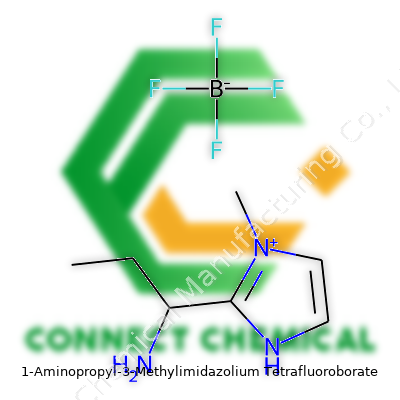
1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate: A Deep Dive
Historical Development
Back in the late twentieth century, researchers started turning away from traditional volatile organic solvents and began exploring ionic liquids as safer, more versatile alternatives. Among these, 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate (often dubbed [APMIM][BF4] in the lab) emerged as an answer to growing interest in green chemistry and more stable, tunable solvents. Chemists in the 1980s and 1990s pushed the boundaries, linking imidazolium frameworks to functional groups and pairing them with different anions. As the field of ionic liquids boomed, this specific molecule drew attention for its impressive chemical stability and tailorability, ideal for a lab looking to cut hazards and improve extraction yields. By the early 2000s, this compound started showing up in academic articles that tackled catalysis, carbon capture, and advanced separation processes.
Product Overview
1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate comes out of the bottle as a clear or slightly yellowish liquid, sometimes carrying a faintly amine-like odor. Not just another salt, it brings together the amphiphilic, charged imidazolium core and a propyl amine arm, paired with a tetrafluoroborate anion, forming a combination that dissolves a wide range of organic and inorganic materials. Labs have leaned into this product for its balance between hydrophilicity and hydrophobicity, making it unusually good for solubilizing both polar and nonpolar compounds. Compared to classic solvents, this one rarely evaporates, reducing air quality risks and making it easy to recover and recycle.
Physical & Chemical Properties
Pouring the liquid yields a viscosity that’s higher than water but much lower than molten salts. This ionic liquid stays fluid even at room temperature, resisting crystallization down close to -40°C. It won’t boil off at the high temperatures typical in catalyst-driven reactions, with a decomposition point up above 250°C for most commercial grades. Water content changes its performance, so buyers look for product specs listing water below 0.1%. Chemically, the amino group offers sites for hydrogen bonding and phase transfer, leading analysts to use this compound for specialty separations. Its ionic nature grants it almost zero vapor pressure, making spills less worrisome—though that same property calls for careful wastewater handling.
Technical Specifications & Labeling
Suppliers usually bottle it in amber glass to avoid light-driven degradation. Labels provide CAS number 946538-16-7, a molecular formula of C7H14BF4N3, and detail minimum purity—most grades clock in above 99%. Users look for tight controls around moisture and halide impurities, scanning certificates of analysis for conductivity and color. Safety sheets call out eye and skin irritancy, and quality assurance teams track batch numbers, storage history, and shelf life. Sometimes, lot-specific pH and density get listed to match process requirements in pharmaceuticals, catalysis, or research labs.
Preparation Method
In most research and industrial settings, synthesis kicks off by alkylating 3-methylimidazole with 1-bromopropylamine under reflux, coaxing the formation of the functionalized imidazolium cation. The next step swaps the bromide with tetrafluoroborate via metathesis, often using silver tetrafluoroborate as the agent, producing a highly pure salt after filtration, careful washing, and vacuum drying. Analytical chemists rely on NMR, FTIR, and ion chromatography to confirm structure and rule out leftover starting materials. Scaling up usually involves closed reactors with inert atmospheres, protecting both product integrity and worker safety.
Chemical Reactions & Modifications
The real star on this molecule’s resume lies in its ability to act as both solvent and active participant in reactions. In organic synthesis, the amino group attaches quickly to aldehyde- or ketone-containing substrates, building imine intermediates without the water sensitivity of traditional amines. The imidazolium ring can stabilize transition states in catalytic cycles, and the tetrafluoroborate anion stays mostly inert, avoiding unwanted side-reactions. Some R&D groups modify the side chain—swapping propyl for longer or branched alkyls, or adding functional groups—for performance tuning in separations and electrochemical applications.
Synonyms & Product Names
Depending on the supplier or research publication, this compound goes by several names: 1-Aminopropyl-3-methylimidazolium tetrafluoroborate, APMIM BF4, N-(3-aminopropyl)-3-methylimidazolium tetrafluoroborate, and sometimes AIMIM BF4. Reading product catalogs, the key traits to check are presence of the aminopropyl group and the BF4 anion, since mislabeling or abbreviation mistakes can lead to confusion with non-functionalized imidazolium salts.
Safety & Operational Standards
Long shifts using new ionic liquids always demand attention to safety. Even though APMIM BF4 doesn’t burn or release flammable vapors like classical solvents, the presence of fluorinated components means thorough ventilation and nitrile gloves are part of any good practice, since skin contact can cause irritation or sensitization. Labs keep eye wash stations nearby, and anybody handling the compound for scale-up or process work wears goggles and protective clothing. Wastewater disposal follows strict guidelines, since fluorinated wastes can persist and bioaccumulate. Storage means sealed bottles in cool, dry spots, with logs maintained for inventory checks and shelf-life monitoring.
Application Area
Scientists and engineers push this chemical into several frontiers. In analytical chemistry, its ability to break down stubborn solutes helps labs extract precious metals and organic toxins from tough matrices. For electrochemistry, it delivers wide electrochemical windows, enabling better energy storage and conversion. Some groups build it into supported ionic liquid membranes, separating gases or removing dyes and pharmaceuticals from water streams. As a reaction medium, it enables more selective and higher-yielding syntheses in pharmaceutical and polymer research. Industrial adoption remains slow mostly due to cost and complex recycling needs, but researchers keep finding clever ways to justify exploring it instead of old-school solvents.
Research & Development
Research teams see this compound as a platform, not just a tool. Synthesis optimization has trimmed energy inputs and reduced precursor waste. Engineers working with process intensification have used APMIM BF4 in microreactors, boosting reaction rates and reducing byproducts. Environmental chemists keep probing how to clean up spent ionic liquids, focusing on degradation pathways that won’t produce harmful fluorinated fragments. Material scientists have published on embedding APMIM BF4 into nanoporous supports, hoping to construct more stable, selective membranes for long-term operation. As more funding moves into green chemistry and carbon management, journals keep filling up with new approaches to utilizing and modifying this versatile compound.
Toxicity Research
Every new chemical draws scrutiny around safety, and APMIM BF4 is no exception. Animal studies point to low acute toxicity by ingestion or skin exposure compared to more caustic amines, but chronic or high-dose effects haven’t been fully worked out. Environmental toxicologists worry about persistence when it reaches waterways, especially considering the stability of the tetrafluoroborate anion. There’s ongoing interest in designing rapid screening tests to predict bioaccumulation. On the shop floor, the main hazards come from skin and eye contact, so staff take exposure seriously and report symptoms quickly. Over the past decade, more data has emerged showing cautious optimism, as the compound’s low volatility gives it lower atmospheric impact—but regulatory agencies continue pressing for complete toxicity breakdowns, especially before considering approval in consumer-facing applications.
Future Prospects
Looking ahead, demand for more sustainable solvents grows as chemical industries face stricter rules and higher environmental standards. APMIM BF4 stands poised to play a bigger role in clean-tech applications, including capture of carbon dioxide and selective separations in wastewater treatment. Newer manufacturing methods might ease cost barriers, with some groups developing greener routes using renewable raw materials and less aggressive reagents. If advances in recycling and end-of-life handling keep pace, market leaders could start replacing hazardous solvents in pharmaceuticals, fine chemicals, and batteries with ionic liquids like APMIM BF4. The main challenges revolve around environmental persistence and large-scale economics, but growth in academic research guarantees a steady flow of ideas and incremental improvements. Every gain in process efficiency, energy savings, or waste reduction helps make a case for broader adoption, and scientists are not running out of ways to push this molecule to new frontiers.
Why Chemists Care About Ionic Liquids
Working with chemicals for years, I’ve seen interest grow around ionic liquids. These are not your average solvents. Take 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate, for example; it stands as an ionic liquid that manages to handle so many modern-day laboratory headaches better than most classic compounds. At room temperature, it remains liquid, and that helps people avoid issues tied to many dangerous and smelly organic solvents. That’s a big draw for anyone who’s spent hours in a poorly ventilated lab.
A Push for Greener Chemistry
Green chemistry keeps popping up in research goals, forcing a closer look at how chemicals are made, used, and tossed out. 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate has picked up attention because it doesn’t catch fire like some older solvents, doesn’t evaporate quickly, and breaks down less in the air. In other words, it reduces waste. Fewer fumes see the air, and fewer toxins hit the water systems. The EPA and several international organizations have published strong evidence linking old-fashioned organic solvents to environmental and health problems. So, something as stable and manageable as this ionic liquid gives researchers a way to clean up both lab routines and finished products.
Industrial Impact
Ionic liquids shake things up in industry. Whether it’s pharmaceuticals, refining oils, or specialty polymers, companies look for efficiency and flexibility. 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate brings to the table an ability to dissolve a surprising range of substances. For drug manufacturers, that means smoother reactions and sometimes purer medicines. Chemical engineers appreciate that it keeps things going with fewer breakdowns or shutdowns, especially in processes demanding fancy separation or purification. A study from the Royal Society of Chemistry points out that using this ionic liquid can sometimes cut costs and energy in extraction steps—a clear win for producers.
Limitations and the Roadblocks
No chemical solution sits free of issues. Sourcing some of the ingredients and scaling up from a beaker to industrial vats challenges even the best teams. Prices for specialized chemicals fluctuate, making it hard for small operations. Waste handling still matters, since not every ionic liquid breaks down quickly in the environment. If we keep pushing these non-traditional solvents, it calls for stronger guidelines—both to protect workers and to make sure the waste doesn’t pile up downstream.
Looking Ahead
Experience says clever teamwork between academia, manufacturers, and regulators brings the strongest improvements. Some research groups keep looking for even less toxic tetrafluoroborate alternatives, learning from both successes and mistakes. With the wider use of green solvents like 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate, the hope grows for safer workplaces, smarter chemistry, and less pollution over the long haul. If the chemical industry keeps listening to those with hands-on experience and keeps pulling data from real-world trials, expect to see even more practical and safe uses for these liquids soon.
Digging into the Risks
Most folks working in a lab know the routine: gloves on, splash goggles tight, not a whiff of lunch anywhere near the fume hood. Still, every substance brings its own quirks. With 1-Aminopropyl-3-methylimidazolium tetrafluoroborate—call it an ionic liquid for short—questions pop up again and again. Is it really as safe as some say, or is there more to the story?
What Science Tells Us
Plenty of texts call ionic liquids “green.” Usually, that means they evaporate a lot less than old-school organic solvents. This cuts back on air emissions, so breathing in large amounts of vapor probably won’t happen as easily. The “tetrafluoroborate” part can give people pause. Fluorine in chemicals isn’t new—think Teflon or refrigerants. Still, a few compounds in this category can break down into harmful by-products like hydrofluoric acid (HF).
From what chemists and safety officers say, the aminopropyl-methylimidazolium cation doesn’t bring massive toxicity, but data on chronic exposure run thin. Eyes, lungs, and skin, as with many ionic liquids, may tingle or sting after contact. Sure, it’s less flammable than acetone or ether, but “less flammable” doesn’t mean “risk-free.”
What Experience and Research Show
Lab hands often rely on safety data sheets (SDS), and this one usually lists irritant for skin and eyes, sometimes harmful if swallowed. Real-world handling backs this up. Getting a drop on a finger without gloves leads to redness and itching—one reason for the PPE drill. A few researchers tested biodegradability and aquatic toxicity; tetrafluoroborate-based ionic liquids don’t break down easily, raising concerns for wastewater disposal. In my own years of prep work in university labs, the rule always boiled down to: “treat any unknown liquid with as much respect as an old mercury thermometer.”
Weighing the Consequences
People want to avoid substances that linger in the environment or get into waterways. Throwing leftover solutions down the drain doesn’t cut it. When ionic liquids like this one escape, the tetrafluoroborate anion may stick around, presenting a potential hazard for aquatic life or for water treatment.
The limited data on long-term health effects stops me from calling it completely safe. Repeated exposure, especially if the substance gets on bare skin or splashes into eyes, risks irritation. If you’re heating or reacting this chemical above room temperature, decomposition looms larger—dealing with corrosive off-gassing like HF puts safety on the line.
Smart, Practical Strategies
Working with new or less common chemicals, taking it slow matters more than bravado. In a lab or plant, basic steps save trouble: gloves (nitrile or similar), lab coats, decent eye protection, and good ventilation form a reliable base. Handling spills with absorbent pads—catch and contain before cleaning thoroughly—follows the golden rule of “don’t touch, don’t inhale.” If disposal feels uncertain, consult with environmental safety staff, not the kitchen sink.
Research continues on improving the safety record for ionic liquids, and educators push for more transparent handling guidance. Anyone handling 1-Aminopropyl-3-methylimidazolium tetrafluoroborate owes it to themselves and their coworkers to respect gaps in knowledge and keep up with new studies.
Better Safe than Sorry
Respect for chemicals didn’t start with this generation, but stories of careless handling tend not to end well. 1-Aminopropyl-3-methylimidazolium tetrafluoroborate doesn’t leap off the bench as the biggest menace. Still, using practical caution, grounding decisions in research, and sticking to responsible disposal keeps both people and the planet on the right side of safe.
Getting to Know the Structure
Chemistry often brings some mouthful names, and 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate is no exception. Stripped down to the basics, we're looking at a molecule with two main parts: an organic cation and an inorganic anion. The cation takes the form of a modified imidazolium ring—imagine a five-membered ring with two nitrogen atoms and three carbons. Calling it “1-Aminopropyl-3-Methyl” tells us what’s hanging off that ring. At the first position, you’ve got a three-carbon aminopropyl group (CH2-CH2-CH2-NH2), stuck right onto the nitrogen. At the third position, there’s a methyl group (CH3).
All this gives a charged, flexible molecule, ready to interact with a range of other structures. Now, for the counterion: tetrafluoroborate, BF4-. This part consists of a boron atom surrounded by four fluorine atoms, balanced and compact.
Why This Structure Matters
The fused elements in this ionic liquid shape the story. The aminopropyl chain offers new chemistry you won’t find in a typical imidazolium liquid. It brings hydrogen bonding and additional reactivity. These properties have caught the eye of researchers for separating gases, catalysis, and manufacturing technology that leans on cleaner reaction media.
I’ve seen this kind of compound stepping quietly into labs looking to swap out classic organic solvents. Picture greener chemistry with less volatile emissions and custom-tailored properties. The charged head of the imidazolium ring gives stability, but the aminopropyl arm brings the action: The terminal amine captures CO2 or grabs hold of other small molecules in a reaction flask.
Real-World Use: Challenges and Opportunities
Interest in these ionic liquids comes from their non-flammability, low volatility, and ability to dissolve a range of materials. These features can make working in organic labs safer, and the risk of hazardous fumes drops. When tasked with separating tricky mixtures or pulling precious metals from wastewater, scientists need a material that doesn’t break down in harsh conditions. Here, the sturdy tetrafluoroborate anion earns its keep.
Yet, not all is perfect. Some ionic liquids still show toxicity in aquatic environments. Producers and regulators face a balancing act: the benefits of reducing chemical volatility against the possible long-term effects once these molecules leave the lab bench. In my own work, I’ve watched folks grow excited by the unique properties in the reaction flask, but move more slowly as discussions around environmental risk catch up.
Moving Forward: Practical Solutions
More research can help fine-tune these compounds. Swapping amine group lengths or tinkering with the counterion could boost both safety and function. Creating detailed lifecycle assessments will help guide responsible development, so the industry avoids trading one environmental worry for another.
With a good structural understanding, chemists can ask the right questions about how small changes impact big-picture outcomes. By staying hands-on and open to change, the field keeps pushing toward more practical, cleaner chemistry—step by tested step.
Why Storage Choices Matter
Chemicals like 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate aren’t just ingredients in a lab; they’re often the quiet backbone for a lot of progress, from material science to pharmaceuticals. Few people give a thought to what happens outside the experiment. Most accidents I’ve seen started not during experiments, but because someone shortchanged that humble moment of "Put it away." Safe storage simply saves headaches—literally and legally.
Understanding the Substance
This compound belongs to a group called ionic liquids. Many labs rely on them for their remarkable stability, low vapor pressure, and ability to dissolve a range of substances. Still, even stable compounds need some respect. Tetrafluoroborate salts can react with water, eventually releasing toxic boron-containing byproducts or hydrogen fluoride, both of which spell trouble fast. Last year, an unmarked bottle left under a leaky shelf in my old university developed crusty deposits—turns out those deposits were as nasty as they looked.
Temperature and Environment
Nobody likes walking into a hot, stuffy storeroom, least of all sensitive chemicals. Keep 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate cool—think regular room temperature, certainly below 30°C—and steady. I’ve learned not to stash it over a radiator or near sunlight. Even in climate-controlled rooms, direct light brings little temperature spikes that push chemicals to degrade gradually.
Humidity often sneaks up on even a well-run lab. Water and ionic liquids don’t play nice, often leading to hazy liquids or compromised reactions. Tighten the cap and check the seal often, especially after busy days. Invest in a desiccator for long-term storage, or at least use drying tubes if a desiccator isn’t available.
Containers and Labeling
There’s no glamour in glassware, but the right bottle matters. Use clean, dry glass or polyethylene containers—clear or amber, as most ionic liquids don’t need shielding from light unless listed. Never reuse bottles with even a hint of residue. A wiped-down bottle beats a stuck cap, every day. I once thought a “small” mix-up was harmless, right up to the point where an old solvent reacted with a trace contaminant and made the whole shelf reek of fish.
Label every bottle with more than its name. Date received, date opened, initials, and even a quick hazard summary help when the lab rotates staff or a graduate student moves on. A clear, bold label turns a risky guess into straightforward handling.
Handling Spills and Disposal
Even careful hands slip. For small spills, soak up using inert materials like vermiculite. Avoid water directly, as it swings open the door for unwanted reactions. Bag up waste, and don’t toss down the drain. Local guidelines—not distant theory—rule disposal. Working with safety officers isn’t bureaucracy; it’s a partnership. In my experience, the better you document each bottle, the less trouble you’ll get if an inspection happens.
Building Better Habits
Storing specialty chemicals means thinking ahead. It’s easy to cut corners, especially during crunch times. Clear procedures, regular checks, and honest communication shape a culture of safety—not perfection, but steady improvement. Years of working in both cramped teaching labs and large research facilities taught me that storage isn’t glamorous, but every careful step supports everything that comes next.
Looking at an Unusual Chemical: More Than a Mouthful
If you take a chemist out for coffee, odds are they’ll bring up ionic liquids at some point. Most people out in the world never hear about 1-Aminopropyl-3-Methylimidazolium Tetrafluoroborate, but researchers in labs across the globe have shelves lined with dark vials of the stuff. This compound landed on my radar about ten years ago when I managed a summer internship buried in the basements of a university chemistry department, stuck somewhere between sticky beakers and trembling centrifuges.
Why Researchers Keep Returning to It
Unlike traditional organic solvents, this ionic liquid doesn't catch fire easily, nor does it leave behind the same choking fumes as things like acetone or ether. You don’t end a day in the lab wheezing or worrying if you’ll pass out on the train home. And that alone has drawn countless chemists, including my mentors, to put their trust in it. One postdoc told me, "I could spill a milliliter and not clear the whole lab."
Scientists always keep their eyes peeled for new solvents that don't poison the planet or themselves. Several studies, including a few from universities in Germany and the US, show ionic liquids built from structures like this one can cut down on both emissions and the sheer volume of waste generated.
Catalysis and Separations: Where Magic Happens
In organic synthesis, this compound shows up in reaction mixtures where researchers aim for better product yields, fewer by-products, and lower energy bills. I once saw a team handle pharmaceutical intermediates in an ionic liquid. Their reactions finished faster and gave purer results—without intricate purification afterwards.
Their roles extend far past test tubes: industrial setups use these ionic liquids to strip metal contaminants from wastewater streams, extract rare metals from electronic scrap, or break down biomass into useful chemicals. Real payoffs appear in developing more efficient recycling routes, cutting down on toxic extractants and harsh acids. I’ve followed a project that used this exact imidazolium derivative to pull copper ions from printed circuit boards. No noxious vapor clouds; just a neat separation and recovery with less mess.
Electrochemistry: Batteries and Beyond
One area where companies put real money behind research comes in batteries and supercapacitors. With high electrochemical stability and solid ionic conductivity, this chemical supports novel electrolytes in both lithium-ion and next-generation battery designs. I talked with engineers trying to lower the risk of fires in consumer electronics. They replaced volatile carbonate solvents with these viscous, stable liquids—and saw measurable drops in thermal runaway incidents.
It also helps polish up metal electrodes or even plate thin coatings of metals under greener conditions. The past two decades give plenty of evidence that this approach not only reduces environmental tolls but sometimes outperforms the status quo.
Hard Lessons and Real Bottlenecks
All that said, the path from lab bench to factory floor isn’t a straight walk. Cost and recyclability create tough hurdles. Commercial production sits at a higher price point than old-school solvents, and recycling ionic liquids without losing their unique properties keeps researchers awake. My old professor used to say, “You can spend a fortune on one flask of progress.”
Pushing this compound further into industrial mainstream means smarter design for lifecycle use, regulations that reward less toxic practices, and more corporate commitments to sustainable chemistry. Historical lessons show that safer, more creative choices start small—but solutions that save on both cleanup and conscience grow over time, if we let them.