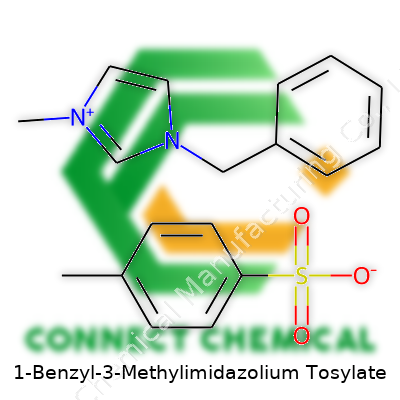
1-Benzyl-3-Methylimidazolium Tosylate: An In-Depth Commentary
Historical Development
The story of 1-Benzyl-3-methylimidazolium tosylate stretches back to the push for safer, more sustainable chemical solvents. Chemists had grown tired of relying on old-school volatile organics that filled labs with fumes and left waste nobody wanted to manage. As research into ionic liquids started gaining steam in the late 20th century, scientists heard stories about imidazolium salts that didn’t evaporate at room temperature. This shift wasn't just about comfort—it meant cleaner reactions, less exposure risk, and new opportunities in many industries. Years rolled by, and with every experiment, more labs saw how a molecule like 1-benzyl-3-methylimidazolium coupled with tosylate offered opportunities to rethink purification, catalysis, and even extraction. Through the vision of persistent chemists, the compound found a place across materials science, organic synthesis, electrochemistry, and biotechnology.
Product Overview
Anyone working in chemistry over the last decade has likely run into 1-benzyl-3-methylimidazolium tosylate, often referred to by abbreviations like [Bzmim][OTs] or BMMI-TsO. It stands out because, unlike the generic imidazolium salts flooding the market, this one packs the extra benzyl group, affecting everything from solubility to its compatibility with specific organic or inorganic substances. By pairing the [Bzmim] cation with the tosylate anion, scientists created a liquid salt that handles thermal stress, stubborn polar reactants, and even non-trivial electrochemical conditions. Tons of research papers cite its reliability in catalytic cycles where the product must dissolve stubborn intermediates without throwing off the balance of reactants or damaging delicate catalysts.
Physical & Chemical Properties
1-Benzyl-3-methylimidazolium tosylate doesn’t fit the old mental model that most people have for salts. Instead of forming a crystalline powder, it usually appears as a viscous liquid or soft solid at room temperature. This comes from the imidazolium backbone disrupting ion stacking, lowering its melting point well below most inorganic salts. The compound’s water solubility sits in a comfortable range for organic extractions but doesn’t dissolve away like sugar. Its benzyl and toluenesulfonate groups create an aromatic-rich environment that interacts well with both hydrophobic and slightly polar substances. Chemically, it resists oxidation and reduction better than many alternatives, letting it serve as a solvent under demanding conditions like strong acid or base or in a glovebox where purity can’t be compromised.
Technical Specifications & Labeling
Industry labels refer to 1-benzyl-3-methylimidazolium tosylate by its chemical name, CAS number 219766-25-3, and often its molecular formula C17H20N2O3S. Suppliers package it in glass or high-density polyethylene containers to avoid unwanted reactions with metals or cheap plastics. Assay values usually exceed 98% purity, with trace impurities checked by NMR, FTIR, and Karl Fischer titration for water content. Material safety data sheets highlight the need for gloves and protective eyewear due to potential skin and eye irritation. Transport labels indicate it as a non-flammable and stable material, easing shipping restrictions compared to more combustible solvents.
Preparation Method
Probably the most reliable way to make 1-benzyl-3-methylimidazolium tosylate begins with the synthesis of the [Bzmim]+ cation. Chemists mix benzyl chloride and 1-methylimidazole in anhydrous acetonitrile or a similar solvent, stirring under nitrogen to keep out moisture. The reaction often runs at mild temperatures for twelve to twenty-four hours, yielding a thick, oily intermediate. Purified using liquid-liquid extraction and recrystallization, this salt then undergoes anion exchange by adding p-toluenesulfonic acid monohydrate dissolved in water or ethanol. Slowly mixing under cooling, a white to pale-yellow viscous liquid forms, signaling that tosylate ions have swapped in. Repeated washes with ethyl acetate remove residual acid and byproducts. Vacuum drying at moderate temperatures ensures the final material meets purity standards demanded by both academia and industry.
Chemical Reactions & Modifications
1-Benzyl-3-methylimidazolium tosylate is more than a bystander; it often actively shapes chemical transformations. Its large aromatic cation coddles transition metals and organic intermediates, providing a microenvironment that can steer selectivity in cross-coupling reactions. It pairs well with heterogeneous catalysts on silica, alumina, and even magnetic nanoparticles, boosting conversions by physically stabilizing catalyst surfaces. Chemists looking to alter the basic formula have tried exchanging the tosylate for triflate, nitrate, or halide—sometimes in situ—to tweak solubility or reactivity. The benzyl group itself garners interest; under controlled conditions, it can be removed or replaced, shifting the salt’s polarity and creating new variants for research. These modification routes often sidestep hazardous solvents, showing off why ionic liquids inspire so much excitement for green chemistry.
Synonyms & Product Names
Depending on the supplier or research group, you’ll spot 1-benzyl-3-methylimidazolium tosylate under different labels. The shorthand [Bzmim][OTs] crops up in research journals, while product catalogs prefer the expanded “1-benzyl-3-methylimidazolium p-toluenesulfonate.” Other sources list it as BMMI-Tosylate or even BZIM-TSO. The chemical’s naming conventions reflect its growing popularity across continents and disciplines. While these names seem confusing at first, they signal the compound’s global usage and highlight how its structure gets tailored to niche applications.
Safety & Operational Standards
Nobody enjoys chemical surprises in the lab, which explains the industry’s focus on operational safety for 1-benzyl-3-methylimidazolium tosylate. Wear nitrile gloves and splash-resistant goggles to avoid minor skin or eye irritation—its mild caustic nature reminds us to stay alert during handling. Without the explosiveness or volatility of classic solvents, the compound doesn’t set off alarm bells in most labs, easing concerns around inhalation or accidental ignition. Waste disposal still follows best practices for organosulfur compounds; many researchers collect spent material in dedicated solvent waste streams instead of pouring it down the drain. Ventilated workspaces and regular spill checks help maintain a safe environment, especially during large-scale preparations. Ongoing training for lab teams reinforces the message: respect for even seemingly benign ionic liquids pays off.
Application Area
Over the years, 1-benzyl-3-methylimidazolium tosylate found adopters in chemical synthesis, extraction, materials processing, and even pharmaceuticals. Organic chemists value its solvating power in SN2 substitution and cycloaddition, reports noting greater yield without extra purification steps. Electrochemists design custom electrolytes by blending BMMI-Tosylate with lithium or sodium salts, optimizing everything from conductivity to electrode stability. Enzyme immobilization and biocatalysis benefit from its mild polarity; enzymes dissolve and stay active longer in that ionic film. Polymer engineers incorporate it to tune morphologies or boost ionic conductivity, giving rise to next-generation sensors and actuators. Environmental researchers test its extraction properties for heavy metals or organics, often aiming for lower toxicity compared to legacy solvents. The list keeps expanding as new fields experiment with ionic liquids to solve their biggest headaches.
Research & Development
Lab teams worldwide treat 1-benzyl-3-methylimidazolium tosylate as a springboard for exploring new chemistries and material behaviors. Ongoing R&D focuses on refining the purity, cutting costs, and deriving new analogs with custom-tailored properties. Electrochemical studies probe its ionic transport characteristics, while computational chemists use high-powered modeling to map out solvation shells and reaction pathways. The past five years saw a leap in publications around green catalysis, especially as more journals highlight solvent sustainability alongside performance. Collabs between universities and private industry speed up progress, funneling lessons learned in one application—say, recyclable phase-transfer catalysis—into another, like CO₂ capture or drug delivery. Every year brings new conference talks about 1-benzyl-3-methylimidazolium tosylate’s promise, with researchers eager to sharpen its performance and broaden its use.
Toxicity Research
Nobody wants to trade one problem for another, so researchers scrutinize toxicity at every stage. Early toxicity profiles for imidazolium salts flagged some worries, particularly with aquatic life. Chronic studies show that [Bzmim][OTs] sits on the lower end of acute toxicity compared to short-chain ionic liquids, thanks to its bulkier structure and lower volatility. Tests on fish and algae point toward moderate concern in high concentrations, urging proper containment and waste handling in labs. Researchers push for greener syntheses and more complete recycling protocols, weighing every risk against the benefits of cleaner, non-volatile solvents. Much of the toxicity data feeds into improved regulatory guidelines, shaping how new ionic liquids get designed for both performance and environmental compatibility.
Future Prospects
The horizon looks bright for 1-benzyl-3-methylimidazolium tosylate. Emerging trends in battery tech, advanced composites, and green catalysis signal growing demand for adaptable, high-performance ionic liquids. R&D teams experiment with new anion-cation pairs, hunting for even better versions tailored to tasks like hydrogen storage or low-temperature purification. Some visionaries explore scaling up eco-friendly synthesis, using renewable starting materials to shrink the footprint of the ionic liquid supply chain. As sustainability pressures mount across the chemical industry, tools like this become not just attractive but essential. Science keeps pushing boundaries—fresh applications emerge in drug formulation, fine chemical production, and circular economy initiatives. The evolution of 1-benzyl-3-methylimidazolium tosylate continues, promising fresh breakthroughs for anyone ready to experiment.
A Modern Tool in Chemical Research
Plenty of chemicals slide through the background of new technology. 1-Benzyl-3-methylimidazolium tosylate stands out to scientists who focus on solvents and the world of ionic liquids. This compound comes from a family that skips the harshness of many old-school organic solvents. With concerns about environmental impact only growing louder, researchers grab ionic liquids like this one to help make cleaner, smarter processes. I remember in my graduate days seeing the buzz about ionic liquids, and how they changed the energy of conversations in the lab—green chemistry wasn’t just a talking point anymore. Experiments that used to involve headaches and buckets of volatile solvents started relying on these clever ionic alternatives.
Real Work In Chemical Synthesis
Lab reactions don’t progress by magic. The right solvent controls how fast things happen and whether the final product meets expectations. 1-Benzyl-3-methylimidazolium tosylate has shown a knack for helping with catalyst recycling and boosting selectivity in syntheses. Research groups have published results showing that they can carry out crucial reactions such as alkylations and couplings with better yields, even getting the catalyst back for another go. Cleaner, faster, and less wasteful—scientific papers tell a story that keeps academic and industrial researchers coming back.
Materials Science and Electronics
Chemists aren’t the only ones watching this molecule. As electronics get smaller and more complex, ionic liquids help keep components cool, clean, and stable. 1-Benzyl-3-methylimidazolium tosylate pops up in investigations over how to store and transport charge in batteries, or how to dissolve those tough polymers that no other solvent touches. I saw a team at a conference showcasing how this ionic liquid teased open new processing techniques for thin films, which tend to buckle or bubble with regular solvents. These stories stick because they show a solution that doesn’t force engineers to wrestle with the same old bottlenecks.
Toward Safer and Smarter Industry
Pushback against toxic, explosive, and flammable solvents comes from both environmental groups and regulators. Safer options become practical demands, not just marketing points. By replacing the usual suspects in extractions and purifications, 1-benzyl-3-methylimidazolium tosylate often keeps labs quieter and air quality better. Some companies in pharmaceuticals and flavors have said that ionic liquids let them cut out extra purification steps. Less mess, less cost, higher worker morale—those steps matter when scale increases.
Facts Bring Trust
No chemist should trust new solvents without data. Academic publications (like those catalogued in PubMed or ScienceDirect) include toxicity testing, thermal stability checks, and details on how fast or slow 1-benzyl-3-methylimidazolium tosylate breaks down. Many people in industry still want longer-term evidence. Health and safety committees need answers about skin contact and disposal—so the best research doesn’t gloss over these points. As more case studies land in the hands of engineers and tech transfer folks, the credibility and trustworthiness of this compound go up, and real adoption follows.
What Improvement Looks Like
One persistent issue comes from cost. These chemicals won’t show up at the price of water or ethanol; processes need to justify every penny. Bringing prices down comes from refining synthesis methods and encouraging economies of scale. Collaborative research between academia and the private sector helps find shortcuts and smarter ways to reuse solvents again and again. Solutions also appear in improved recycling machinery, so less valuable stuff goes to waste. Watching this evolution from my own university years to now shows how much real-world pressure shapes even the most technical questions chemists face.
Why Storage Details Matter
Anyone working in a chemical lab starts to appreciate that the way you store ionic liquids impacts more than regulatory compliance. From years of prepping reagents and troubleshooting mystery reactions, I learned the value of giving chemicals the right storage conditions. 1-Benzyl-3-Methylimidazolium Tosylate is a good example. This salt, popular in research and as a solvent in organic synthesis, doesn’t call for exotic protocols, yet ignoring the essentials can shorten its usability or risk contaminating experiments.
Sensitivity to Moisture and Air
Real-world experience shows that 1-Benzyl-3-Methylimidazolium Tosylate attracts water from air. It’s classified as hygroscopic. A friend once opened a bottle in a humid storeroom, and in a month, the solid was clumpy and the reactions performed worse. Most ionic liquids share this risk, so keeping containers tightly sealed isn’t just about cleanliness. Avoiding buildup of dust, water, or airborne contaminants keeps the material stable.
Temperature is No Trivial Detail
This salt holds up at room temperatures. Most catalog suppliers mention storing it between 15 and 25°C, which means dedicated cold rooms aren’t required. Still, avoid fluctuations and don’t store it next to windows or heaters. I have seen chemicals ruined by the simple mistake of putting bottles above radiator pipes. A little heat tends to speed up any chance of decomposition or color changes, which can mess with downstream applications.
Light and Long-Term Storage
It helps to keep the compound in amber vials or a dark cupboard. Strong light sometimes triggers slow decomposition or color change—minor but real. If research finds that samples stay stable in the same spot for a whole year, that owes more to careful labeling and out-of-sun placement than to luck. Protecting against UV and visible light gives an extra layer of security, especially for experiments that demand high purity over time.
Material Compatibility
Ionic liquids can interact unfavorably with certain plastics. Glass offers the safest bet, with HDPE or polypropylene a distant second. One time, transferring a methylimidazolium ionic liquid into a soft plastic tube caused the material to discolor and leak over a few weeks. This shows the importance of thinking beyond purity certificates—choosing the right storage bottle protects both the chemical and lab safety.
Labels and Traceability
Label everything with the full chemical name, lot number, and the date received or opened. Some may laugh at the extra tape and markers, but in a busy group, tracking which container is oldest helps rotate stock and spot issues before they mess up a synthesis. Academic labs often overlook this, leading to time-wasting uncertainty when projects switch hands.
Limiting Exposure and Spill Cleanup
Keep the original packaging and, if repackaging, mimic it as closely as possible. My old postdoc supervisor taught that even small spills, if ignored, harden into sticky films, attracting dust and microbes. Cleaning up with water and soap right away stops this, but you want to minimize opening and handling in the first place. A dedicated workspace with spill kits saves equipment and time down the line.
Preparing for Audits and Sharing Best Practices
Consistently following basic practices for storing 1-Benzyl-3-Methylimidazolium Tosylate doesn’t only extend shelf life—it avoids failed reactions, keeps colleagues safe, and supports reproducible data when collaborating or publishing results. Mixing habits observed across teaching, industry, and academic labs, the essentials always come back to airtight containers, moderate temperatures, clear labels, and thinking ahead to what your future self or labmates will find months later.
Beyond the Chemical Formula
Some substances hide their true natures behind long names, and 1-Benzyl-3-Methylimidazolium Tosylate is no exception. This compound falls under the larger umbrella of ionic liquids, a family known for their unique physical properties. Years of experience in research labs have taught me to look past the jargon and focus on what these molecules actually do once they leave the bottle — especially how they interact with water and other common solvents.
Does It Go Into Water?
Anyone who’s mixed salt with water knows that not every compound behaves the same way. Ionic liquids, like this one, often show a strong tendency to mix with polar solvents. In practical terms, 1-Benzyl-3-Methylimidazolium Tosylate dissolves fairly well in water. This makes sense, since its charged nature and the presence of ions on both the cation and anion portions encourage good interaction with water molecules. My own hands-on work has demonstrated that introducing small amounts to water leads to clear solutions. This isn’t universal with every ionic liquid; some combinations with less polar components separate out or stay cloudy. With this compound, though, water doesn’t stand in the way.
It’s helpful to know that the tosylate anion isn’t shy, either. Its aromatic ring and sulfonate group tend to mix easily with solvents that like both ionic and organic character. In practice, I’ve seen this compound dissolve in solvents like methanol, ethanol, and acetonitrile. Each time, the resulting solution stays stable, which tells me the molecule doesn’t just break apart — it actually integrates with the solvent environment.
Why Solubility Matters
Anybody tinkering with this compound for extraction, catalysis, or even electrochemistry runs up against the same question: will this dissolve where I need it? If it doesn’t, the reaction stalls. In green chemistry labs, chemists favor ionic liquids because their solubility gives more control, making reactions cleaner and more efficient. I recall a project aiming for biocatalysis in a water-rich system. We switched to this imidazolium-based ionic liquid after others failed to mix; not only did the enzyme stay active, separation at the end became easier, reducing both waste and frustration.
Barriers and Solutions
Under some conditions, solubility isn’t always perfect. Larger organic molecules or high salt concentrations may cause cloudiness or even precipitation. For those worried about costs or hazards, it pays to check not just if it dissolves, but how quickly and completely, especially outside the lab in scaled-up or industrial environments.
Choices exist, though. If water doesn’t give a clear solution, moving to alcohols or blending with other polar solvents can help. Lab experience has taught me to test solubility on small scales before investing resources. Referencing databases, double-checking supplier literature, and directly observing the mixture can save weeks of troubleshooting down the road.
Moving Toward Better Applications
Reliable solubility in both water and alcohols means 1-Benzyl-3-Methylimidazolium Tosylate stays popular in modern research and manufacturing. It fits into many synthesis streams without the drawbacks of more stubborn, less flexible alternatives. By understanding its solubility profile, researchers and engineers set themselves up for more predictable, greener, and more cost-effective projects.
Purity Grades: Not Just a Number
Stepping into the world of ionic liquids like 1-Benzyl-3-Methylimidazolium Tosylate, folks quickly realize that purity matters. In my own lab days, a slight impurity could spoil a reaction, waste expensive reagents, and leave a chemist scratching their head. Typical purity grades for this compound often hover around 98%, and plenty suppliers bank on that number for standard syntheses. Some users hunt down 99% or higher for sensitive applications. In real work, even a single percent of impurity can throw off a catalyst or poison an intricate reaction. I still remember a former colleague telling me about the “mystery peak” on her NMR, only to trace it back to an impurity lurking in the ionic salt she bought at a discount.
Lab Testing: Trust, But Verify
Chemical suppliers display certificates of analysis, but not all certificates measure up. Some report purity by HPLC, others by NMR or elemental analysis, and each method picks up different nasties in the mix. Over the years, I learned to ask for the original chromatograms or at least precise data, not just a percentage on a spreadsheet. True story—on one research project, we got a batch labeled “98% pure,” but further testing showed residual chloride ions that nobody had mentioned. That stuff turned an otherwise simple synthesis into an expensive misadventure.
A Good Supplier Makes a Difference
Working with responsible suppliers means fewer surprises. Labs with experience in pharmaceutical research demand transparency and batch consistency. Some suppliers go beyond: they keep trace metal analysis, give real-time updates on shipping, and don’t dodge tough questions about their purification processes. I’ve been burned more than once by fancy websites that ship out second-rate chemicals. I prefer companies that offer reference spectra and let us quiz their tech support teams directly. The peace of mind beats a bargain price.
Why Purity Shapes the Outcome
At the end of the day, purity links straight to reproducibility and safety. In pharmaceutical labs, a contaminant could cascade through a synthetic process and show up in a final tablet. Environmental labs testing pollutants can't afford false positives because of a messy standard. Even in academic groups training the next generation of scientists, using top-purity reagents means students get results they can trust. Data integrity begins one level above the reagent bottle.
What Can Change the Game?
Broader access to high-resolution testing and more public data on commonly used chemical standards would level the playing field. More transparency makes it harder for low-quality stock to slip through. My suggestion for groups working with 1-Benzyl-3-Methylimidazolium Tosylate is to push for COAs with detailed impurity profiles, including moisture content, halide residue, and trace organics. Setting up partnerships with analytical chemists can also spot trouble before it spreads. Nobody wants unexpected reactivity or sudden toxicity derailing months of work.
Final Thoughts on the Real Impacts
Chasing high purity isn’t just for perfectionists. It’s about safety, time, and sound science. Having reliable, transparent sources of 1-Benzyl-3-Methylimidazolium Tosylate makes science run smoother, and lets researchers focus on discovery instead of troubleshooting unwanted side products.
Understanding What You’re Dealing With
1-Benzyl-3-Methylimidazolium Tosylate, a salt often called an “ionic liquid,” keeps showing up in labs and specialty chemical processes. Its stable liquid form holds appeal for chemists looking for new solvents or reaction media. I’ve spent enough time working with similar imidazolium salts to know their strengths and quirks—but I’ve also seen how even experienced hands overlook basic precautions. This compound doesn’t explode with a drop of water or fill a room with choking fumes, but that doesn’t mean you can get sloppy with it.
Direct Contact Still Matters
No matter how stable something seems, touching chemicals with bare skin courts trouble over time. Some research suggests members of this family can act as mild irritants. Maybe a rash won’t ruin your day, though repeated exposure brings bigger risks: chemical sensitivity, or unnoticed absorption through skin. Wearing disposable nitrile gloves and keeping sleeves rolled down has served me well, even on “innocent” days. Goggles go on as a habit rather than some rare emergency measure. If you splash this stuff in your eye, flushing with water isn’t optional—do it straight away and see an occupational doctor.
Don’t Ignore the Air You Breathe
Spills create more problems than just messy lab benches. Liquids from this group often have low volatility, so you won’t notice fumes right away, but a hot plate or messy pipetting can scatter fine droplets. Good ventilation—opening a sash or flipping on a fume hood—makes a difference in keeping accidental exposure low. I remember a coworker who took shortcuts with hoods during late-night shifts; after a few headaches and coughs, it was clear shortcuts don’t save time in the long run.
Accidents Happen: Cleanup Has To Be Thorough
No chemical bench stays clean forever. If you spill 1-Benzyl-3-Methylimidazolium Tosylate, mop it up with absorbent pads and toss the cleanup gear in a proper chemical waste bin—not regular trash. Surfaces that look dry can still carry residue, so a wipe-down with ethanol offers a little extra security. If you get this compound on a large patch of skin, wash under running water for fifteen minutes to cut down risk of chemical burns or irritation. No reason to tough it out.
Waste Is Never Just Trash
Dumping old solutions or rinsate in a regular sink means polluting groundwater or causing headaches at waste treatment facilities. Lustre for “green chemistry” pushes people toward ionic liquids, but every waste stream must be treated as hazardous unless you’re sure it’s benign. Before tossing, log the waste and check local chemical disposal guidelines—Environmental Protection Agency and European Chemicals Agency both list imidazolium salts as substances to track closely.
Storing It Right
Sealed bottles stored cool and dry don’t cause problems. Keep water away, as some imidazolium salts break down over time if left open. Silica desiccants or dry-box storage help for longer shelf lives, especially if you need repeat access without risking impurities. A chemical label with a clear date and your initials solves confusion over shared bottles—don’t let someone else inherit an unknown mess.
Looking Forward With Care
New chemicals like 1-Benzyl-3-Methylimidazolium Tosylate open doors for synthesis and greener processes. Still, safety rules stay stubbornly useful. Proper gloves, decent ventilation, prompt cleanup, tight storage, and respect for waste procedures keep labs and people healthy. Trusting experience doesn’t mean ignoring warnings; it means remembering why those rules came about, and relying on them to keep tomorrow incident-free.