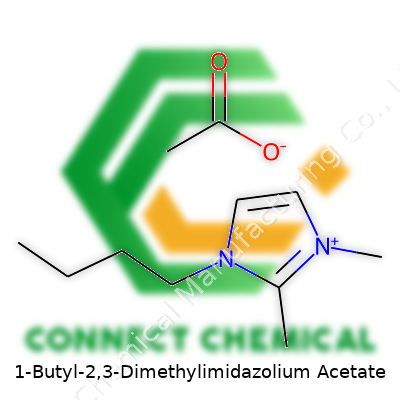
1-Butyl-2,3-Dimethylimidazolium Acetate: A Close Look at a Transformative Ionic Liquid
Historical Development
The evolution of 1-Butyl-2,3-Dimethylimidazolium Acetate followed the surge in research on ionic liquids that began to gain momentum in the 1980s. Back then, scientists hunted for alternatives to volatile solvents that had burdened labs and factories with environmental and safety headaches. The imidazolium family grew out of this search, with chemists tuning both the cation and anion to fit different needs. 1-Butyl-2,3-Dimethylimidazolium Acetate popped up not as a laboratory curiosity but from a genuine push to find task-specific ionic liquids with low volatility, thermal resilience, and a knack for dissolving biomaterials. Over several decades, this compound transitioned from an academic interest to practical use, marking another step in the story of green solvents.
Product Overview
1-Butyl-2,3-Dimethylimidazolium Acetate stands out as a colorless to pale yellow, viscous liquid. Its ionic nature gives it stability where traditional solvents break down. The butyl and dimethyl substitutions on the imidazolium ring provide a balance between hydrophobic profile and solubility, especially when working with both organic and inorganic samples. Packed in glass or high-density polyethylene containers, its purity levels—upward of 98%—meet requirements for most lab and industry applications. Cost fluctuates with purity and the source of the precursors, often depending on the stringency of quality controls adopted during manufacture.
Physical & Chemical Properties
This ionic liquid sports a melting point below room temperature, usually staying in liquid form except in the coldest conditions. Its density hovers around 1.09 g/cm³. Water solubility remains strong, leading to hygroscopic tendencies. It resists vaporization—good news for workers tired of breathing fumes. Thermal stability stretches over a wide range, often tolerating temperatures above 150°C without significant decomposition. The acetate anion contributes weakly basic properties, a point that comes into play during cellulose processing. Conductivity falls in the range common for ionic liquids, making 1-butyl-2,3-dimethylimidazolium acetate suitable for electrochemical setups. Viscosity tends to be higher than many solvents, challenging when mixing at scale.
Technical Specifications & Labeling
On the shelf, bottles bear labels outlining CAS numbers, hazard warnings, batch identifiers, and material purity. Labs appreciate clear instructions for storage—cool, dry, tightly sealed to combat air and moisture exposure. Transport regulations often treat this compound as non-hazardous by global standards, but documentation ensures nobody skips safety steps. Certificate of Analysis (CoA) comes standard with high-grade stock, especially when destined for regulated environments.
Preparation Method
The usual synthetic route kicks off with the alkylation of 2,3-dimethylimidazole using butyl halides to yield the imidazolium salt. This step requires dry solvents and controlled heat to minimize side reactions. After salt formation, metathesis with sodium or potassium acetate replaces the initial halide anion with acetate. Washing and careful drying follow to rid the product of by-products and excess reagents. For truly clean output, vacuum distillation or multiple recrystallizations step in. Yields reach respectable levels—about 70–90% if attention stays sharp throughout. The route scales modestly, attracting small and medium industrial outfits.
Chemical Reactions & Modifications
Beyond faithfulness as a solvent, this ionic liquid plays a role in catalysis and as a reaction medium. The acetate’s basicity encourages esterification and transesterification reactions, unlocking efficient biomass conversion. The cation tolerates functional group modifications, sometimes lengthening the butyl chain or exchanging methyl groups for other alkyls, each new version adjusting properties like water affinity or solvent power. Under reaction conditions, 1-butyl-2,3-dimethylimidazolium acetate often emerges unchanged, though at high temperatures or with strong acids, degradation products, such as imidazole derivatives, may crop up. Understanding such limits has shaped where the compound finds its safest and most efficient uses.
Synonyms & Product Names
Researchers bump into this liquid under slightly different tags, the most common being [BM2MI]Ac or BMIMmAc. Chemical suppliers selling into specialty markets use names close to these or rely on systematic terminology for paperwork purposes. Those searching for safety data sheets, regulatory submissions, or academic articles learn to watch for variations based on regional naming conventions.
Safety & Operational Standards
In the lab, direct skin contact should be avoided because it can cause mild irritation. Some ionic liquids, especially those with longer alkyl chains, pose bigger risks for skin permeability or bioaccumulation but evidence places 1-butyl-2,3-dimethylimidazolium acetate on the lower side of the risk scale. Inhalation exposure rarely worries most users because the vapor pressure stays low, yet gloves and eye protection aren’t negotiable. Disposal raises the question of environmental persistence. Ionic liquids can resist biological degradation. Disposal through incineration—under controlled conditions—remains the preferred route. Following local chemical and environmental guidelines when disposing of residues or rinsing glassware prevents accidental release into water systems.
Application Area
In biomass processing, this solvent tackles cellulose dissolution, pushing boundaries in making biofuels and bioplastic precursors. Traditional cellulose solvents usually need tough or toxic environments to break down plant fiber. Here, a bath of 1-butyl-2,3-dimethylimidazolium acetate manages the task at gentle temperatures and with relatively limited corrosion. It shows promise in electrochemistry, providing stable conductive media for battery or capacitor research. Analytical chemists favor it for challenging separations and extractions, particularly for molecules that shrivel up or degrade in standard organic solvents. Material science teams use it to prepare gels and composites from otherwise uncooperative biopolymers.
Research & Development
Academic and industrial researchers still investigate how this compound interacts with different substrates, striving to expand its usable applications. Teams test new catalytic processes with 1-butyl-2,3-dimethylimidazolium acetate, measuring improvements in yield and selectivity against time-honored benchmarks. Most progress appears in eco-friendly transformations—cellulose to simple sugars, lignin fractionation, and the development of greener plastics. Multinational projects approach the solvent with high-throughput screening and computational modeling. Every leap forward finds a champion ready to compare results against other ionic liquids, mapping where this compound excels and where competitors hold the edge.
Toxicity Research
Long-term toxicity studies tell a nuanced story. Short-term exposures, both in animals and through environmental simulation, show minimal acute toxicity. Chronic exposure data remains sparse, though the scientific consensus supports careful use due to some ionic liquids’ role in aquatic toxicity and slow degradation. Waste from industrial processing requires special care to avoid contamination of wastewater streams. As more countries scrutinize persistent organic pollutants, additional tests around reproductive toxicity and biodegradability will push developers toward safer, more degradable variants. Investing in green chemistry at the R&D level offers real value for both compliance and peace of mind.
Future Prospects
Future demand for ionic liquids leans on environmental regulations and consumer appetite for green alternatives. Industries looking to cut hazardous solvent use spot opportunity in 1-butyl-2,3-dimethylimidazolium acetate. Its success depends on solving issues of cost and recovery, especially since large-scale applications need robust, closed-loop recycling or highly selective cleanup techniques. Researchers have started developing techniques for catalyst recovery and solvent recycling that promise to make the economics more attractive. Advances in renewable resource sourcing and circular economy models underpin these efforts. If these challenges are addressed, this ionic liquid stands poised to lead not just in chemical processing but in any field requiring safer, more sustainable solvent systems.
What Makes This Chemical Interesting?
Years of tinkering in labs have shown that some chemicals quietly change how industries run. 1-Butyl-2,3-Dimethylimidazolium Acetate belongs to a family called ionic liquids. Unlike water or oils, it doesn’t jump up and evaporate at normal temperatures. This alone opens plenty of doors for chemists and engineers. Sitting down with colleagues in materials science, I've found skeptics turn curious once they see real results—less waste, safer conditions, and new ways to break down stubborn materials.
Tough Jobs: Dissolving Biomass
Few folks outside the science world talk about dissolving cellulose—the stuff plant cell walls are built from. Traditional solvents fall short or chew up too much energy. This ionic liquid breaks cellulose apart efficiently, letting people turn trees or straw into sugar molecules without harsh chemistry or sky-high temperatures. The National Renewable Energy Laboratory points out that some ionic liquids, including 1-Butyl-2,3-Dimethylimidazolium Acetate, help bring renewable fuels closer to reality. In a world after cheap oil, getting fuel from plants ranks high on the list of urgent goals.
Safer Solutions for Industry
I spent two summers in a research group focused on green chemistry. Our team kept bumping into solvents that caused health concerns or pollution headaches. Many ionic liquids steer clear of these problems. Some, like this acetate, stay stable, safe, and even reusable after several cycles. Fewer fumes mean workers breathe easier, and disposal doesn’t wreck water supplies. Labs and companies already deploy such alternatives to meet stricter environmental rules, especially in Europe and Japan.
Enabler for Recycling and Reuse
Those who’ve worked in plastics or electronics see a major roadblock: traditional solvents can’t untangle complex polymers or tricky composites. 1-Butyl-2,3-Dimethylimidazolium Acetate steps up where others fizzle out, helping break down recycled materials so they can return as new products. The Centre for Sustainable Chemical Technologies in the UK lists ionic liquids as key for closing the loop on waste. Here, every small gain in recovery translates to less landfill or incinerator use—an underappreciated step in slashing our environmental footprint.
Smoothing Out Production Hurdles
If you ask any synthetic chemist about annoying bottlenecks, speed and purity always come up. This acetate speeds up certain reactions or removes stubborn byproducts that foul up the next stage. I’ve watched teams at a pilot plant shave days off timelines by swapping in a custom ionic liquid. Sometimes, the cost of the chemical pays for itself through higher output and lower downtime. That kind of boost means a lot in pharmaceuticals or specialty chemicals, where delays knock millions off the bottom line.
Room for Improvement
Wide adoption always stirs up trade-offs. Right now, one of the hurdles is producing this ionic liquid without high cost or sketchy feedstocks. More sustainable manufacturing, perhaps using waste CO2 or renewable alcohols, would help. Teams worldwide explore recycling schemes for used ionic liquids so nothing gets lost down the drain.
Building on a Strong Foundation
Taking a step back, I see 1-Butyl-2,3-Dimethylimidazolium Acetate as a bridge to safer, cleaner chemical processes. Its unique mix of stability, safety, and versatility lines up well with the push for greener industry. My hope mirrors many in applied science: more research, more practical tweaks, and fair policies can turn this lab curiosity into an everyday tool for cleaner technology.
Getting to Know the Stuff
Stepping into the world of ionic liquids, 1-butyl-2,3-dimethylimidazolium acetate doesn’t exactly roll off the tongue or sell itself at first glance. The clear to pale yellow liquid slips through your fingers slicker than vegetable oil. Its faint smell hints at vinegar mixed with old books—thanks to the acetate. One big draw: It doesn’t really boil, even if a lab hotplate gets wild. Instead, it starts breaking down after about 220°C, sidestepping the risks that come with highly volatile organic solvents.
If you knocked over a beaker, you’d notice it doesn’t evaporate the way alcohol or acetone does. Its low vapor pressure means those fumes won’t sneak up on you in a small lab, a real bonus for safety. The liquid keeps itself stable at room temperature, resisting efforts to freeze solid unless your lab cools down to minus ten Celsius. I’ll admit, I learned this by propping it up in an unreliable fridge during grad school. It stayed, stubbornly, a puddle.
What’s Going On at the Molecular Level
1-Butyl-2,3-dimethylimidazolium acetate (let’s call it [BMMIM][OAc] from here) owes its slick behavior to the ionic structure. Instead of molecules bumping around, you get positively and negatively charged bits—cations and anions—hugging and tussling. That charge lets [BMMIM][OAc] dissolve a surprising range of chemicals, especially anything polar. Toss in some cellulose—hard wood pulp, waste from the farm—and [BMMIM][OAc] eats away at the tight bonds holding everything together.
This chemical can act a bit like a chameleon. Tweak the imidazolium ring or swap the acetate for another group, and the liquid’s entire demeanor changes. But acetate brings a special kick: It loves picking apart tough biomolecules. Chemists chasing better biofuels or plastic replacements turn to [BMMIM][OAc] because it can unravel plant fibers that most liquids ignore.
Why Properties Like This Matter
Industry depends on solvents that won’t disappear or leave behind toxic residues. The water-miscible nature of [BMMIM][OAc] means it prefers dissolving in water over oil, which makes washing out leftovers easier and safer for downstream use. Labs trying to recycle solvents see real cost savings; this stuff doesn’t vanish into thin air, so you can reuse it. In my own experience with green chemistry, the ability to drop waste and keep things closed-loop brings both peace of mind and fewer headaches for compliance.
Beyond the bench, there’s a growing demand for better ways to break down natural waste. [BMMIM][OAc] stands out for processing lignocellulosic material—think corn stalks, wheat straw, or forestry leftovers—into sugars and other molecules that can lead to bioethanol or bioplastics. Researchers have published studies showing [BMMIM][OAc] pulls off this trick more efficiently than many ionic liquids, in part because of that acetate bite. According to several peer-reviewed articles (including ones shared by the American Chemical Society), yields for glucose after cellulose dissolution rise by as much as 70% compared to traditional acid hydrolysis.
What to Watch Out For
The move toward green chemistry brings questions. Some ionic liquids prove tricky to make and expensive at scale. The environmental impact isn’t always straightforward—production can still create waste, and not every process using [BMMIM][OAc] is closed-loop. Recovery methods, such as vacuum distillation or anti-solvent addition, bring challenges. Labs and plants working with [BMMIM][OAc] need to keep an eye on costs, purification steps, and proper recycling. In our pilot facility, clogging and contamination cropped up when trying to recover the liquid for a third cycle, showing scale is no small matter.
Solving these hitches starts early: Investing in purification, exploring less energy-hungry recovery, and designing new reactor systems all help. Collaborative projects between universities and industry partners have begun to pay off. With the right approach, 1-butyl-2,3-dimethylimidazolium acetate opens the door to safer, cleaner, and more efficient industrial chemistry.
What Is This Chemical?
Most folks outside specialty chemistry labs have never crossed paths with 1-Butyl-2,3-Dimethylimidazolium Acetate. It pops up in conversations about ionic liquids and specialty solvents. In the lab, engineers and researchers reach for it to dissolve cellulose or run experiments that demand something more exotic than old-fashioned organic solvents.
Hazardous or Not?
Worries about chemicals usually come down to two big questions: Are you likely to run into this stuff? If you do, will it hurt you? Research shows 1-Butyl-2,3-Dimethylimidazolium Acetate doesn’t belong among the most notorious toxins or volatile solvents. It does not evaporate easily at room temperature, so there’s less worry about breathing in big clouds of gas from it. Still, it isn’t “harmless.”
Studies on similar ionic liquids give mixed results. Some are hard on skin, irritating to eyes, or show mild toxicity to cells under the microscope. There’s not a pile of long-term studies tracked on this specific compound, but related chemicals sometimes pose toxicity hazards to aquatic life. Safety Data Sheets tell workers to use gloves, goggles, and good ventilation. I’ve seen folks ignore glove warnings once or twice, but chemical burns and rashes bring them around fast.
Why It Matters
People have a growing curiosity about what lurks in lab and industrial chemicals. My time in university research labs showed me how quickly carelessness leads to painful lessons. Even low-to-moderate toxicity compounds can cause real trouble over months or years if handled sloppily. The difference often comes down to knowledge and respect—the first splash or spill can make a skeptic into a glove devotee overnight.
Early on, I learned it’s wise never to trust a chemical just because it isn’t famous for causing cancer. There’s a big gap between “no evidence of cancer risk” and “safe for daily use without protection.” Regulatory agencies often lag behind emerging science. Without decades of health data, experts recommend caution with anything new, including ionic liquids. A compound’s novel properties might end up helping the world, but that doesn’t give users a pass on responsibility.
Protecting People and the Planet
Stories of new, greener solvents circulate in scientific circles. Some ionic liquids claim to do less environmental harm than traditional solvents, but each formula carries its own baggage. Persistence in soil or water, or toxicity to micro-organisms, may crop up later. I’ve heard biologists raise the alarm after seeing lab waste slow down seed germination.
Mitigation comes down to well-worn habits: proper labeling, sealed containers, and targeted waste streams. Labs and manufacturers need to keep safety training up-to-date—not just dusting off handouts from years past. Smart policies treat every chemical as a potential hazard until proven otherwise. Substituting less hazardous materials, improving venting, and investing in waste capture all remain essential for modern research.
Building a Safer Culture
No measure beats education. From freshmen to seasoned chemists, reminders about the hidden dangers of unfamiliar compounds keep complacency out. Open access to toxicity data, clear safety signage, and honest conversations between managers and staff all help. Real safety isn’t about blind fear, but about building experience, sharing hard lessons, and not underestimating what a single spill can do.
Getting Familiar with the Substance
1-Butyl-2,3-Dimethylimidazolium acetate looks harmless at first glance, almost like any clear, viscous liquid you'd find in a lab or a chemical storeroom. But its chemical makeup demands respect. Forgetting that can bring accidents, spills, and headaches nobody wants to clean up. This compound plays a big part in some cutting-edge work, especially in processing cellulose and creating cleaner industrial systems. Its benefits shine brightest when folks treat it with the same care as any potentially hazardous chemical.
Not Just Another Bottle on a Shelf
People tend to stash chemicals wherever there's space, but this one's got specific needs. Humidity will wreck its quality. Water creeping in leads to product breakdown, and that undermines scientific results, upsets budgets, and introduces unpredictable hazards. Based on my time in shared research spaces, nothing creates tension like a mislabeled or poorly sealed bottle leaking across shelves. So, the solution stays simple: keep containers tightly closed, store them in a dry place, and pick a cabinet or shelf away from sunlight and heat sources.
Lab workers may forget the risks after routine sets in. That complacency opens doors to mishaps. For instance, storing 1-butyl-2,3-dimethylimidazolium acetate near strong acids, bases, or even oxidizers builds a recipe for disaster. Mixing incompatible chemicals by accident can trigger hazardous vapors or reactions. Chemicals do not forgive laziness or shortcuts. Secure the container in an area set aside for ionic liquids or organics, and label with the date received and date opened.
Personal Safety Starts with the Basics
Direct contact with skin or eyes turns a busy workday into a visit to occupational health—the chemical has a knack for causing irritation. Gloves (nitrile, not latex), long sleeves, and safety glasses knock down the most common risks. In research groups I’ve worked with, the biggest error comes from overconfidence: “It’s just a liquid.” One splash can remind everyone that this isn’t a game. Even after years in the lab, I reach for my gloves by habit; it isn’t paranoia, it’s good practice.
If a spill happens anyway, don’t just grab some paper towels. Ventilate the space and tackle cleanup with absorbents meant for chemicals—no homemade improvisation. Once done, waste gets sealed in designated containers, then handled by professionals who know the regulations. This keeps employees, facilities, and the environment safer.
Keep Track, Keep Safe
Digital inventory systems help track storage conditions and remind people to check on bottles regularly. I’ve seen research halted because a needed chemical degraded in storage, not because anyone wanted that to happen but because nobody kept tabs. A periodic review—checking labels, seals, and expiration—lets small issues stay small.
Suppliers provide detailed safety information for a reason. Relying on those safety data sheets beats guessing and avoids costly mistakes. Each person in the chain, from storage to use to disposal, plays a role in protecting themselves and their community. In the end, a little care with 1-butyl-2,3-dimethylimidazolium acetate keeps projects moving forward and turns the lab from a risk zone into a safe and productive space.
Why Purity Matters in the Lab and Beyond
Anyone who’s spent time in a chemistry lab knows how fast an experiment can go off the rails with impurities in the mix. I’ve seen small impurities throw off data so much that weeks of preparation end up in the trash. In my experience, working with 1-butyl-2,3-dimethylimidazolium acetate, or BMMIM Acetate, really drives home the point: purity affects everything from reaction yields to equipment lifespan to reproducibility.
With this compound, the purity question isn’t academic. It shows up in the numbers: research labs look for purity of at least 98%, and many suppliers provide 99% or even 99.5%. That little half percent counts. Trace water, leftover reactants, or unmentioned byproducts turn routine synthesis into a gamble. Trusted analytical reports rely on proper HPLC, NMR, and mass spectrometry to prove the material stands up to spec.
In talking with colleagues handling this ionic liquid, the most common issues come from trace water or halide contamination. A little extra water can throw off solubility, while invisible chloride means corrosion just around the corner. So every batch they're buying, they're questioning the documentation, the storage, and the actual numbers on the certificate. Sometimes, you just don’t learn these things until the column strips stop working or glass joints seize tight with a weird white film.
What to Expect from Packaging
Not every lab is running year-round, full-tilt syntheses—people want flexibility. Suppliers typically pack this material in tightly sealed bottles made of amber glass or high-density polyethylene. These bottles can be as small as 25 grams, perfect for pilot work and specialized assays. Bulk buyers often grab 100 grams, 250 grams, or even up to 1 kilogram. For industrial runs or regular research programs, standard packaging scales up to 5 kilograms or more, often with more robust containers to fight off air and light exposure.
Tight seals are more than good practice: this liquid can suck up water from the air and spoil before anyone uncaps the bottle. Sometimes you see vacuum-sealed or argon-flushed vessels, especially from suppliers who ship globally. Over the years, I've unwrapped more than a few dusty, foil-wrapped packages just to get to a tiny vial inside a double-sealed bag. Some might roll their eyes at the overkill, but just one ruined experiment from a soggy ionic liquid fixes your attitude fast.
Where Quality Slips and How to Fix It
Quality control rests on more than just a certificate from a supplier. I've always double-checked with my own Karl Fischer titration for water, just because surprises happen. Good suppliers answer questions and share batch data. Poor ones dodge or delay—I've learned to respect that warning sign. For research teams, pooling trusted supplier lists and sharing experiences helps steer new folks away from problematic sources.
Clear labeling and consistent excipient lists also play a role. Suppliers who are upfront save everyone time. There’s also a push to improve tamper-evident packaging and transparency in shipping histories. Years ago, delivery trucks sat in the sun too long and the product arrived “off.” Now, tracking and reporting have started to close that gap. Scientists and technicians need that confidence to move fast and publish reliable work.
Better Choices Fuel Better Science
After years working with specialty chemicals, I know nobody gets excited about solvents and reagents—until a mistake messes up a project. That's when the conversation about purity and packaging matters most. Stay picky. It builds better science, protects equipment, and saves everyone in the long run.