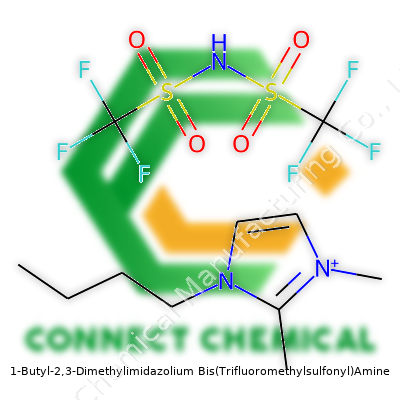
1-Butyl-2,3-Dimethylimidazolium Bis(Trifluoromethylsulfonyl)Amine: A Deep Dive
Historical Development
Long before labs buzzed with the acrid tang of ionic liquids, chemists experimented with molten salts for electrochemistry and metallurgy. Then, researchers in the late 20th century saw a path forward: imidazolium-based ionic liquids. They decided to trade the bulky, cumbersome setups for something nimbler. In this history, 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)amine—often known by the shorthand BMMI-NTf2—showed up with a set of properties that shook up laboratory protocols. The push for safer, more stable solvents ran parallel with the green chemistry movement, when regulatory changes started clamping down on volatile organics. Chemists, tired of the same flammable, toxic solvents, tried BMMI-NTf2 and realized it didn’t behave like the old guard. It shrugged off air and moisture and brought a range of uses no naphtha or acetonitrile could match. This wasn’t a happy accident; materials science had been angling for a solution to high-temperature engineering challenges. The early focus on room-temperature ionic liquids—particularly ones with imidazolium cores—set the stage for BMMI-NTf2, which hit the scene as a robust tool for every lab bench seeking stability and creativity.
Product Overview
BMMI-NTf2 is not the most famous molecule outside chemistry circles, yet its market has stretched across specialties. Its colorless to pale yellow appearance and liquid nature at room temperature (melting point below 25°C) have led to rapid adoption in analytical labs and as a medium for chemical synthesis. The two methyl groups on the imidazolium ring affect viscosity and reduce melting point, compared to earlier analogs. With a butyl tail, the compound resists water yet dissolves a wide range of salts and organics. In my own experience, swapping out DMSO for BMMI-NTf2 in organic reactions cut down on noxious fumes and improved product isolation. Instrumentation techs told me it gave them fewer headaches, because leaks or small spills didn’t mean evacuating the lab.
Physical & Chemical Properties
This compound remains a liquid under typical lab conditions, with a density hovering between 1.4 and 1.5 g/cm3. People working with it rarely worry about volatility—the vapor pressure sits comfortably low, nearly eliminating operator exposure. Its remarkable thermal stability stands out, tolerating sustained exposure well above 200°C without breaking down, and even higher for short bursts. I’ve seen it used with reactive metals, oxygen-sensitive compounds, and moisture-sensitive reagents, all without the frantic glovebox shuffling usually required. Its hydrophobic character and negligible miscibility with water allow it to extract neutral and charged analytes from aqueous phases efficiently, a real leap forward over classic hydrocarbons.
Technical Specifications & Labeling
Suppliers typically offer BMMI-NTf2 as a high-purity, water-clear liquid packaged in glass bottles lined with PTFE to resist acidic or basic degradation. Specs on most safety data sheets list purity above 99.0%. Trace halides, transition metal contaminants, and water content should remain well below 50 ppm, as even these small amounts can poison downstream catalytic processes. Solubility data include high affinity for acetonitrile, dichloromethane, and aromatic hydrocarbons, with negligible solubility in methanol and water. Temperature-stability charts and electrochemical windows are always marked, since many users build batteries or sensors requiring precise limits. Proper labeling must highlight the corrosive action against certain plastics, as some storage mishaps have seen oozing containers make a mess of shelves.
Preparation Method
The synthetic route for this ionic liquid builds from imidazole chemistry, often starting with 1-butyl-2,3-dimethylimidazole. Quaternization using alkyl halides produces the required cation. Introducing lithium bis(trifluoromethylsulfonyl)imide in aqueous media triggers a metathesis reaction, forming BMMI-NTf2 with a dense, oily layer separating from brine. Proper drying over phosphorus pentoxide strips stubborn moisture, and further filtration ensures near-complete purity. Technicians who handle scale-up must vent off small amounts of volatile amines and sulfur compounds, as those carry over from commercial reagents. Over time, pilot plants reduced waste by recycling the saline byproducts, a practice praised by environmental audit teams.
Chemical Reactions & Modifications
In synthetic chemistry, BMMI-NTf2 acts as a solvent and a participant, able to facilitate nucleophilic substitutions, phase-transfer catalysis, and electrochemically driven transformations. I watched colleagues run metal-catalyzed cross-coupling in BMMI-NTf2, producing high yields for compounds that usually demand more aggressive solvents. Its thermal window and resistance to oxidation let chemists push reactions harder, sometimes eliminating the need for crowd-control in the form of excessive cooling or nitrogen atmospheres. Modifying the imidazolium ring swaps or tacks on new side chains, creating a family of ionic liquids tailored for specific conductivity or extraction challenges.
Synonyms & Product Names
BMMI-NTf2 goes by a knot of technical names in catalogs and articles: [BM22], 1-butyl-2,3-dimethylimidazolium bis(trifluoromethanesulfonyl)imide, and its CAS number circulates widely. European suppliers sometimes label it as an imide salt while Asian manufacturers stick to NTf2 terminology. Trade names rarely catch on, as end-users want the chemical formula up front; everyone from journal editors to warehouse clerks relies on standardized abbreviations to limit confusion on chemical manifests.
Safety & Operational Standards
Handling BMMI-NTf2 doesn’t mean ignoring basic safety. Protective gloves, splash goggles, and fume extraction remain vital. Chronic skin exposure leaves irritation or mild burns, particularly if unwashed after a spill. Inhalation is uncommon given low vapor pressure, though handling under vacuum or with rough agitation can aerosolize drops. Disposal in regulated waste streams is non-negotiable, given the compounded risk of fluorinated byproducts in groundwater. Industry standards require double containment at volumes above 500 mL and detailed hazard communication on every label. Safety audits in my own laboratory focused on spill containment and the inspection of older containers, as PTFE liners do degrade.
Application Area
BMMI-NTf2 found its footing as an extraction solvent for trace metals where organic and aqueous incompatibilities stymied researchers. Electrochemists took notice because it supports wide electrochemical windows, enabling sensors or batteries that run at voltages many solvents can’t withstand. In organic synthesis, the reduction in flammability risk alone convinced several pharmaceutical companies to include it in early process development. Some materials scientists exploit its low melting point for preparing thin-film membranes and separation layers that stand up to caustic conditions. I’ve heard engineers use it for lubricants in extreme-temperature environments—power stations, high-vacuum gear, and even some aerospace systems benefit from such chemical stability.
Research & Development
R&D poured into ionic liquids over the last two decades because so many industries started needing stable, tunable, and relatively safe solvents. BMMI-NTf2 stands out in the literature for supporting high-throughput catalysis, novel battery electrolytes, non-aqueous chromatography, and anti-static coatings. Reports highlight its amphiphilic balance and nonreactivity with both strong acids and potent reducing agents. In my network, analytical chemists talked about using it to pre-concentrate rare earth metals from mixed solutions. Researchers continue to tweak side chains and blend with other ionic liquids to push boundaries of selectivity, conductivity, and recyclability, making incremental advances that could mean the next leap in battery life or greener syntheses.
Toxicity Research
Initial testing flagged imidazolium ionic liquids as relatively low risk compared to legacy industrial solvents, but scientists remain watchful for chronic exposure effects, especially aquatic toxicity. BMMI-NTf2 shows moderate toxicity to invertebrates and microorganisms in controlled studies, which led to tighter effluent restrictions. Work crews train on safe handling and PPE, and solvent recovery programs kick in even before regulations demand it. Some toxicology reports have surfaced, documenting irritation in mammalian studies and signaling caution about long-term bioaccumulation. Environmental groups called for clearer guidelines and supported intensifying waste minimization strategies.
Future Prospects
Ionic liquids like BMMI-NTf2 will keep drawing interest as pressure mounts to switch from volatile, flammable solvents to stable, greener alternatives. Ongoing innovation in battery and supercapacitor design counts on these materials for reliable, high-temperature operation. As circular economy concepts take shape, efficient recovery and recycling of ionic liquids will be key concerns. Chemical engineers look to scale up sustainable processes using these solvents, and regulatory agencies keep a close eye on environmental performance. With more research into toxicity and ecosystem impacts, safer, even more robust analogs will likely follow. This push for practical, low-risk, and high-performance media means BMMI-NTf2 and its next-generation relatives will not fade from the scene soon.
The Curious Case of an Ionic Liquid
Steps into a modern chemistry lab often reveal a shelf lined with mysterious bottles, each marked with labels nearly impossible to pronounce. One of these, 1-Butyl-2,3-Dimethylimidazolium Bis(Trifluoromethylsulfonyl)Amine, looks straight out of a science fiction novel. At first glance, nobody outside research circles would recognize what it does, or why it matters. Yet, this ionic liquid steadily gains ground for some surprising reasons.
Why Scientists Gravitate Toward This Compound
Chemists face a tough world full of trade-offs. Many older solvents, like toluene or chloroform, linger in the air as health hazards. After years of hearing about cancer risks and ozone trouble, researchers push for green chemistry—less toxic, more sustainable approaches to the same old reactions. That’s where this mouthful of a substance comes in. It doesn’t evaporate easily, so you avoid breathing in nasty fumes, and it comes with a thermal stability regular solvents can’t touch. At the bench, less volatility means less fire risk and safer working conditions.
Handling the Messy Work of Separations
Pulling one compound away from another underpins much of chemistry. In classic labs, separating chemicals involves mind-numbing hours juggling different solvents and temperatures. This ionic liquid can cut down on the slog. For some pharmaceutical extractions, its selectivity offers a shortcut with much higher yields, specifically where water-based or nonpolar solvents fail. Supporting this, academic research regularly points out that the imidazolium part of the compound helps create a stable environment for grabbing target molecules without forcing side reactions.
Powering Energy Tech
Look inside the battery powering your phone or watch, and you’ll spot designs moving away from plain old lithium salt solutions. This compound shows up in studies on next-generation electrolytes. Its ability to conduct ions without degrading at high charge cycles grabs the attention of engineers working on electric cars and stationary energy storage. Scientists praise these ionic liquids for remaining liquid at room temperature, proving non-flammable, and making batteries safer in the event of overheating. A battery with this within the electrolyte doesn’t simply die on you; it’s less likely to explode.
Industrial Hopes and Growing Pains
No chemical is perfect. Long synthesis times and tricky purification routines hold back widespread adoption. Disposal of used solvents also raises concerns if the waste stream isn’t treated properly. Yet, these drawbacks haven’t stopped certain companies from experimenting with this compound for dissolving cellulose, making plastics from plant fibers rather than oil. Companies like BASF and Merck have started to dabble in large-scale trials, trying to balance environmental responsibility and economic sense.
Room to Improve, Space to Grow
Improving cost and supply reliability remains the key. Researchers at European and Asian universities dive into methods for recycling these liquids, cutting out the highest synthetic hurdles. At my own workplace, we tried small-scale purification, using less expensive reagents, which brings the price closer to reality for commercial users. Industry partnerships and open sharing of safety data foster trust and push development along.
The ordinary worker won’t find this chemical in their backyard any time soon, but the quiet work happening in labs could ripple out to better, safer consumer products—greener batteries, safer factories, and even less pollution from pharmaceutical manufacturing. It all begins with dedicated people willing to test, tweak, and believe some tough chemistry is worth the effort.
What’s Going On with Ionic Liquids?
Ionic liquids earn a lot of hype these days. Scientists talk about them as “green solvents” because they hardly evaporate and usually don’t burn easily. So, on paper, these chemicals look pretty safe. My first reaction when I read about ionic liquids was optimism—they sounded futuristic, and I wanted to believe in a cleaner alternative to old-school solvents. Still, once you start using these in a lab or a plant, the basic question never goes away: Do they really avoid the dangers that have always come with industrial chemicals?
Not All Properties Equal Safety
One misconception shows up again and again: just because something has a low vapor pressure doesn’t mean it’s harmless. Ionic liquids still have a mix of cations and anions, and those can cause trouble in our bodies and in the environment. You’ll find plenty of ionic liquids that are tough on the skin or the eyes. Some versions don’t break down easily, so they can hang around in soil and water for a long time. My own lab once tested an imidazolium-based ionic liquid—a family that keeps popping up in research. The results came back toxic to common freshwater organisms. That told me the “green” label really doesn’t fit every sample out there.
What the Data Tells Us About Toxicity
Ionic liquids grabbed a lot of attention partly because big chemical spills have scared folks—think of the 2014 Elk River spill in West Virginia. Workers and citizens both want to avoid another headache like that. Studies do show some ionic liquids have lower flammability than legacy solvents, so the risks of fire drop in industrial settings. But that doesn’t cancel out all other risks.
Research from published journals tells another side of the story. Some imidazolium and pyridinium ionic liquids harm cell membranes at low concentrations. Fish and water fleas exposed to small doses develop health problems or die. The toxicity depends on the specific structure of the ions, so even a tiny change can make a big difference, but there’s no such thing as a totally safe ionic liquid today. I see more labs using zebrafish tests now, chasing down what happens when these chemicals escape into water supply chains.
Protecting Health and the Environment
Nobody in industry wants to introduce new hazards—or even raise the old ones. So, letting the “green” tag guide safety reviews misses what matters: actual data and prudent design. Risk assessments for any ionic liquid have to mirror those for classic chemicals. That means testing for skin and respiratory impacts, as well as aquatic toxicity. You won’t get by with a one-size-fits-all answer here. I’ve watched projects stall because researchers overlooked persistence or bioaccumulation.
Better designs come from considering toxicity from the start. That includes predicting impacts before the first flask of ionic liquid gets produced at scale. European and North American agencies encourage this, but the real improvement shows up when chemists work with toxicologists—not after, but during the early synthesis phase. Rigor in these safety checks helps protect folks on the production line and in downstream communities. The only way we find out what’s safe is by clear, shared data, not buzzwords.
Looking Ahead
Chemical technology keeps pushing forward. New materials like ionic liquids bring big promises, but also the same old safety questions. If we want to call a chemical “non-hazardous,” we need repeatable tests, transparent results, and an approach that values human health and the planet. Otherwise, we trade one set of problems for another in the rush for innovation.
Why Storage Matters More Than Most Think
A lot of products come with long storage instructions buried in the manual or on the back label, but ignoring them can be a mistake. From my experience working in a small warehouse, everyone remembers at least one time when a shipment went bad because the wrong pallet sat by the heater overnight. Taking storage lightly means money lost and safety risked. Temperature, humidity, and even sunlight can throw off quality faster than most folks expect.
Common Sense Meets Science: Keeping Products Safe
Every product brings its own list of demands. Many require temperatures kept cool—medications often lose power or break down if things get too hot or cold. Food can spoil in hours. I’ve seen snacks go stale faster in steamy backrooms. For items like batteries or electronics, moisture is the real enemy. Even the smallest leak in packaging lets humidity sneak in, causing corrosion that ruins an entire lot. No company survives if goods spoil before they reach the shelf. Nobody wants to answer the phone when a customer bites a stale cracker or finds medicine that stopped working.
Security Trust Is Earned, Not Claimed
Anyone handling sensitive or expensive goods can’t cut corners. A secure area—locked, limited access—prevents theft, but also careless damage from visitors who aren’t trained to move things the right way. A coworker once rammed a forklift through shrink wrap, destroying half a pallet of fragile components. One slipup like that costs more than extra training. Even simple records, like logging temperatures and checking seals, add up to big savings in the end.
Proper Packing Makes a Difference
I watched an experienced packer explain to a rookie why bubble wrap and sturdy cartons matter for delicate shipments. He showed how a small crack in a box destroys the shield against dust or pests. For pharmaceuticals, the rule was airtight seals and clean containers. With food, pallets raised off concrete keep rodents out. Details seem small, but neglect turns into big problems. Once, a shipment sat by an open garage door, and mold set in overnight. The cleanup took a week and smelled for longer.
Labeling Isn't Just Red Tape
Labels can seem like a chore. They’re not. Good labels show what goes inside, what conditions matter, and when the product should be checked or moved. Ignoring them leads to lost traceability, which breaks trust with customers and opens up legal headaches. If a recall happens, honest labels turn chaos into a quick, organized process. I learned early that clear writing on boxes is worth more than any software tracking tool—humans read boxes, not barcodes, in a pinch.
Training and Consistency: The Human Element
Machines can chill or heat, ventilate or seal, but people must pay attention, every single shift. Regular reminders, simple checklists, and a culture of asking questions keep everyone sharp. Tools like thermometers and humidity indicators don’t matter unless someone reads them. From the biggest chemical plant to the smallest corner store, the people make or break storage plans.
Building Long-Term Confidence
By setting clear routines, sharing know-how, and staying vigilant, businesses show they respect both the product and the customer. Effort upfront saves far bigger headaches down the line. Storage and handling aren’t glamorous, but they define whether promises made in marketing hold up out in the real world.
Why the Right Electrolyte Makes a Difference
Batteries and supercapacitors seem simple on the outside. Inside, they depend on the chemical dance between electrodes and electrolytes. The electrolyte shuttles ions as energy moves back and forth. Rechargeable lithium-ion batteries, for example, use liquid electrolytes like LiPF6 in organic solvents. These liquids carry ions but can catch fire, raise safety concerns, and degrade over time. Supercapacitors, aiming for rapid charge and discharge, use water-based or organic solutions that operate much like high-speed highways for ions. Choosing the right chemical for this middle layer seems technical, but it shapes the way our phones, cars, and grids store energy every day.
Benchmarks and Barriers in Electrolyte Selection
Not every compound fits what batteries or supercapacitors demand. A good electrolyte stays stable at high and low voltages, does not let electrons through, and dissolves the right salts or acids. Many researchers chase higher voltages to pack more energy, yet face the risk of explosion or breakdown. A candidate chemical must do more than conduct ions quickly; it must survive split-second reversals and harsh temperatures without corroding parts or sparking chemical byproducts.
Performance hinges on details that matter in the real world. In lithium-ion batteries, common liquid electrolytes reach limits when stored hot or pushed to high output. These limits shape why electric cars have strict charging protocols. Aqueous solutions in supercapacitors deliver safer, faster cycles—think grid backup systems needing reliable surges. Water-based solutions lose ground at voltages over 1.23V, yet organic versions handle more, at the cost of toxicity or volatility. I see neighbors weighing EV purchases and asking about fire risks and lifespan—often a simple electrolyte swap behind the scenes makes all the difference.
Evaluating New Chemicals for the Job
Scientists test new chemicals for the job. Room-temperature ionic liquids once seemed promising—non-flammable, stable, tunable—until cost, viscosity, and compatibility hurdles popped up. Research into polymer electrolytes uncovers solid options that cut leakage or safety risks. I recall the excitement when sodium-ion electrolytes showed progress in labs; easier sourcing pushes costs down, yet their lower voltage narrows some applications. Each breakthrough triggers fresh rounds of field testing, showing just how much hands-on scrutiny new chemicals face.
Any would-be electrolyte faces busy workrooms and test stations. Beyond carrying ions, the liquid or solid must not eat away electrode surfaces or deposit unwanted films. Real battery cells get poked, prodded, frozen, and charged until weak points surface. Each generation—lithium, sodium, magnesium—demands chemistry tailored to its own needs. I have seen startups weighed down by materials that looked great in spreadsheets but fizzled when scaled up.
Charting a Path Forward
Pushes for faster charging, safer operation, and lower cost keep electrolyte research lively. Companies, universities, and governments pull together to share test data and real-world lessons. Open data efforts sharpen competition and give small innovators a seat at the table. Clean energy targets, supply chain shocks, and consumer demand all argue for better chemistry that performs in home, car, or grid batteries. In all battery and supercapacitor efforts, real progress comes from tackling materials science head-on—one stable, affordable, and safe electrolyte at a time.
What Purity Means for People and Products
Most folks hear “purity specification” and think about lab coats and endless technical charts. I’ve learned over the years, though, that purity touches almost every part of our lives—from the medicine in your cabinet to the salt on your kitchen table. Purity specification tells you how much of a substance should be the real deal, and how much is made up of things you didn’t ask for. It decides whether a chunk of metal keeps a bridge standing, or a batch of antibiotics fixes what ails you.
Reputable companies stick to these standards for good reason. A hospital, for instance, only buys IV solutions with tightly controlled sodium chloride levels. If that saline solution has extra stuff floating around—oak pollen, or a chemical impurity—the results can turn dangerous fast. In my work with small food businesses, I saw inspections that demanded clear records showing each ingredient’s purity. Stepping outside the lines carried a real risk, both for harm and for reputation.
Testing the Real World, Not Just Numbers
Testing for purity isn’t a guessing game. Labs run tests that compare actual samples to detailed requirements, and there’s no shortcut. For a lot of raw materials—say, powdered Vitamin C—testing starts with high-powered machines like HPLC or mass spectrometry. These machines “sort” each bit of the powder, separating the real stuff from everything else. If regulators say a batch should be 99.8% pure, that means out of a thousand grams, only two can be stray substances.
Testing doesn’t stay locked in the lab. Regular people check for purity, too, even if most don’t call it that. A backyard gardener knows when soil smells off, or water has a bitter taste. That instinct comes from experience, not just science. But when mistakes happen, it’s clear. One time, I watched a whole shipment of dried herbs get tossed out because traces of pesticide showed up during routine purity checks. Nobody got sick, because the testing caught the problem long before those herbs reached a customer’s plate.
Why This Matters and Where We Can Go From Here
Ignoring purity means risk lands on our dinner plates and medicine bottles. Problems multiply fast if companies or regulators loosen standards, or look the other way. Recent food recalls in North America—caused by unexpected bacteria or cleaning agents—came down to failed purity. Some outbreaks ended up costing millions and, more importantly, hurt a lot of families.
We’ve got ways to press for stronger purity checks. People deserve clear labels and open records, especially for what goes into food and medicine. More investment in better, quicker tests can save time and money down the road. During the supply chain chaos last year, some groups pushed hard for more transparency about where raw materials come from and how many steps they take before ending up in stores. It’s not a perfect fix, but it helps set clear expectations.
Demanding higher standards isn’t about chasing perfection—it’s about responsibility. Every time industry leaders or public agencies double down on tight purity checks, they keep goods safer and keep trust intact. That effort pays off—sometimes in ways you see, often in ways you don’t. Purity specification looks dry on the surface, but in the end, it’s about the safety and well-being of real people.