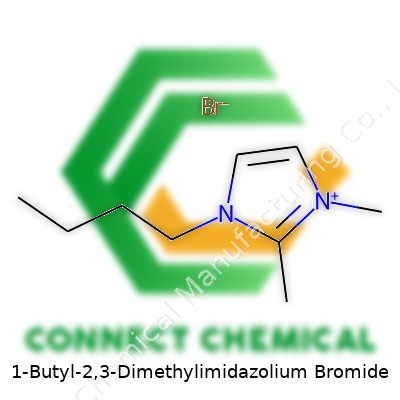
1-Butyl-2,3-Dimethylimidazolium Bromide: Perspective and Commentary
Historical Development
The story of 1-Butyl-2,3-Dimethylimidazolium Bromide draws from a rich tradition of ionic liquid chemistry. Scientists first recognized imidazolium salts as valuable ionic liquids decades ago, thanks to their tunable properties and ability to function in unique environments. The transition from basic imidazolium compounds to designer ionic liquids emerged from persistent effort in both academia and industry to carve out materials that balance stability, solubility, and utility. Over time, methylation and butylation of the imidazolium ring revealed significant boosts in both thermal stability and solubility profiles, providing chemists with a more practical and selective material for numerous lab and industrial applications.
Product Overview
Every time I handle 1-Butyl-2,3-Dimethylimidazolium Bromide, I notice its clean, crystalline form—thanks to the rigors of modern purification and synthesis. Laboratories often select this ionic liquid for its consistent results in organic transformations, electrochemical work, and extraction processes. The bromide counterion plays a critical role here, serving as a strong partner in halide exchange, catalysis, or phase-transfer applications without contributing unwanted reactivity or coloration in the matrix. Researchers tend to favor this salt in comparison to chloride or tetrafluoroborate analogs when selectivity, cost, and ease of separation become key priorities in experimental design.
Physical & Chemical Properties
The compound stands out as a nearly white solid at room temperature, showing off a melting point close to 50°C and decent thermal stability up to around 250°C. The molecular weight clocks in at 275.18 g/mol, and its solubility in water and many polar organic solvents makes it surprisingly flexible for both aqueous and nonaqueous reactions. Viscosity falls into a manageable range, especially compared with more polymeric ionic liquids, so mixing and stirring present few issues in lab-scale synthesis. The chemical structure, featuring long butyl and short methyl groups on the imidazolium core, allows for controlled lipophilicity and hydrophobic interactions within a chemical system.
Technical Specifications & Labeling
Suppliers label the product under rigorous standards to guarantee high purity—often 98% or better—validated by NMR and controlled for halide, water, and heavy metal impurities. Bottles display the chemical structure, CAS number, batch information, and all associated hazard codes for easy auditing and traceability. During every order, it's clear which synthesis lot produced the batch, how long it has been out of cold storage, and if any regulatory restrictions apply for transportation or export.
Preparation Method
Most commercial material comes from a salt metathesis process, starting with 2,3-dimethylimidazole alkylated with butyl bromide in the presence of a base. After reaction, the crude product goes through aqueous workup, drying, and vacuum stripping, sometimes with recrystallization from an alcohol or ether solvent to remove colored byproducts and ensure a neutral pH. In my experience, those last steps make the biggest difference in both shelf life and material consistency, especially if scale-up to kilo quantities is on the horizon.
Chemical Reactions & Modifications
The butyl and methyl substituents limit nucleophilic attack on the imidazolium ring, so the cation stands up well to a range of bases, acids, and oxidants. I've seen the bromide swapped out for PF6-, BF4-, or NTf2- using simple metathesis, creating new ionic liquids for electrochemistry or battery work. Some creative synthetic chemists have even attached functional side chains at the 1-position or performed ring fusions for targeted catalysis. In the right hands, this compound forms the backbone of hybrid materials, such as supported catalysts or polymer electrolytes, broadening its reach well beyond traditional solvent or phase-transfer roles.
Synonyms & Product Names
Common synonyms include BM2ImBr, 1-butyl-2,3-dimethylimidazolium bromide, and Butylmethylimidazolium bromide. Some suppliers truncate the name to BMIM Br, but that risks confusion with the 1-butyl-3-methyl analog—so careful attention during procurement pays off. Tracking synonyms across databases ensures correct risk assessments and shipment documentation for lab audits and regulatory filing.
Safety & Operational Standards
Safety practices for handling 1-Butyl-2,3-Dimethylimidazolium Bromide mirror those for other ionic liquids—mainly gloves, eye protection, and fume extraction to handle any off-gassing or dust. Although the compound lacks strong volatility, it feels oily on the skin and may cause mild irritation after prolonged contact, so prompt washing is a must. Environmental health guides advise limiting discharge into drains or soil, as ionic liquids persist longer in aquatic environments compared to classic organic solvents. Modern safety data sheets from reputable suppliers include acute toxicity studies and detailed spillage procedures, minimizing risk even in case of accidental exposure. Laboratories maintain spill kits, eye-wash stations, and designated storage areas to separate ionic liquids from reactive metals or oxidizers.
Application Area
The range of use impresses even a chemical veteran. Chemists rely on this imidazolium salt for phase-transfer catalysis during nucleophilic substitutions, greener separation methods, and as a stable electrolyte in lithium-ion and emerging battery technologies. Enzyme-catalyzed reactions often benefit from its mild, non-denaturing character—I've seen enzyme half-lives extend noticeably when compared to reactions run in pure methanol or DMSO. Extraction specialists value the material’s unique solvating properties for precious metal recovery from e-waste and ore. In academic circles, the compound serves as a model for structure-reactivity studies on ionic liquid design, with its balance of hydrophobic and hydrophilic features providing testbed data for computational chemistry groups and process engineers alike.
Research & Development
Research continues to push the boundaries—blending 1-Butyl-2,3-Dimethylimidazolium Bromide with other ionic liquids to create task-specific mixtures that tailor physicochemical profiles for each new project. Engineers at both scale-up and bench-level investigate modifications to the cation and anion, seeking reduced toxicity and improved recyclability. Data on ion transport, electrochemical windows, and solvation power influence how this compound finds its way into batteries, solar devices, or designer solvents. Researchers regularly publish new routes for lower-waste synthesis, using greener reagents and better recovery procedures to cut down byproduct loads while increasing overall yield—a focus that mirrors sustainability efforts across the broader chemical industry.
Toxicity Research
Toxicity remains a constant concern. Early studies highlighted the low volatility as a plus, but some ionic liquids accumulate in soil and aquatic systems. Detailed ecotoxicology assays show moderate toxicity to some freshwater organisms, so regulatory agencies caution against large-scale uncontrolled discharge. Luckily, newer studies note that alkyl-imidazolium cations, particularly those with short chains, tend to cause less harm than earlier anticipated, provided labs implement closed-loop handling. Advances in analytical chemistry make tracking trace breakdown products easier, which gives regulators and process safety engineers firmer ground for updating use guidelines and exposure limits. My own experience shows that with careful management, the risk to personnel or the wider environment stays well within industry safety targets.
Future Prospects
The future of 1-Butyl-2,3-Dimethylimidazolium Bromide looks promising as the chemical industry and environmental regulations evolve. There’s clear momentum toward ionic liquids that offer improved safety, easier recovery, and circular use. Next-generation applications in bio-catalysis, energy storage, and advanced separations will likely lean on this unique compound’s stability and tunable nature. Early adopters in green chemistry expect broader adoption in sectors as diverse as pharmaceuticals and electronic waste recycling, helped along by rising data on performance and stewardship. The most significant advances will come from collaborations—academic institutions, regulatory bodies, and manufacturers working in tandem to chart a safe path for this compound’s future.
Better Chemistry Means Cleaner Industry
Over the years, I’ve watched science evolve from chalkboards and bubbling flasks to digital sensors and specialized chemicals with names longer than some novels. One phrase I keep seeing in academic journals and green tech conferences is “ionic liquids.” Among these, 1-butyl-2,3-dimethylimidazolium bromide stands out, not because it promises magic, but because it gives new tools to people who actually spend days trying to solve old problems, not just talk about them.
What’s It Actually Good For?
Industries and labs use this compound for tasks that go well beyond simply mixing things together. Researchers depend on it as a medium for separating chemicals through processes like liquid-liquid extraction. Unlike more familiar solvents such as chloroform or benzene, this salt, at room temperature, doesn’t easily evaporate or ignite. That helps manufacturers shrink health and safety risks and cut back on environmental headaches. After seeing a few chemical plant floors myself, I know the relief a safer substance brings to both management and workers.
One key use comes in catalysis. Some catalysts, particularly those needed for delicate reactions in producing specialty chemicals, need a stable, non-reactive “home base.” The unique set-up of the 1-butyl-2,3-dimethylimidazolium ion lets it carry out this work without interfering in the main reaction. Academic data backs this up, as studies from institutions like the University of York show improved reaction rates and cleaner products when using these ionic liquids. Their use in green chemistry circles keeps growing, and new articles on sustainable solvents often include this compound.
Tackling Industrial Waste and Emissions
Pollution from old-school solvents travels fast and lingers for years. The chemical industry often gets flak for environmental damage, sometimes earned. I remember a client from the midwest who wanted to move away from volatile organic solvents in metal plating. The shift to ionic liquids, including the one under discussion, made it possible to drop air pollution levels and still get the shine and toughness customers wanted. These steps matter because solvents account for about half of hazardous emissions from chemical manufacturing, according to the U.S. Environmental Protection Agency.
Battery and electronics makers value its non-corrosive and stable nature, especially where moisture and temperature swings threaten quality. This isn’t just a boutique solution. Scale-up research through organizations like Fraunhofer and National Renewable Energy Laboratory show real improvements in lithium-ion battery lifespans and efficiency thanks to ionic liquid-based electrolytes.
Challenges and What’s Next
1-butyl-2,3-dimethylimidazolium bromide isn’t perfect. Cost and recycling hold it back from replacing every old solvent or catalyst. Small companies struggle to pay for newer chemicals, so the real edge comes by finding uses where its unique traits really help—like specialized separations, green lab procedures, or electronics work.
The next chapter comes down to collaboration. Partnerships between universities, startups and heavy industry help refine processes, reuse waste, and develop greener, safer variations. Chemical innovation means less trouble downstream—for water, air, and people. As more industries see the benefits for both safety and performance, expect this compound to keep finding new homes in tomorrow’s labs and factories.
The Importance Behind the Formula
Chemistry pulls back the veil on everything from batteries to pharmaceuticals. I’ve always appreciated how a compound’s formula tells a story about its structure and its behavior. For 1-Butyl-2,3-Dimethylimidazolium Bromide, the formula isn’t just a string of letters and numbers. It’s a snapshot of how atoms line up and interact. The formula for this compound is C9H17N2Br. Let’s step back and see what makes each part count.
Why Care About the Details?
Whether you’ve run a synthesis in the lab or just leafed through a bottle of electrolytes, seeing these formulas isn’t just trivia. 1-Butyl-2,3-Dimethylimidazolium Bromide sits in a unique group known as ionic liquids. These salts, which stay liquid at temperatures below 100°C, have found their way into green chemistry, batteries, and even pharmaceutical prep. The imidazolium core, with the butyl and methyl substituents, leads to unique melting points and solvent behaviors.
Fractional chemistry grades in school made it clear: the smallest changes in structure can mess with properties in outsized ways. Swap a methyl for an ethyl or stretch that butyl chain, and a liquid might turn solid, a good solvent becomes a poor one. That’s why every carbon and nitrogen in this formula isn’t just a label — it steers how the molecule gets used.
A Closer Look at the Building Blocks
It’s easy to skip past an imidazolium ion, but it makes a difference. For C9H17N2Br:
- 1-Butyl: Four carbons add flexibility and affect the way ions stack or move past each other.
- 2,3-Dimethyl: The extra methyls tuck onto the ring, changing the electronic behavior, which chemists know turns the dial on how this cation talks to anions like bromide.
- Bromide ion: Not just a balancing act in the formula, but an active player in how the ionic liquid interacts with solutes and surfaces.
Pushing Forward With Ionic Liquids
Lab work brings its own insight—I've watched how different ionic liquids make or break a reaction. Not all are created equal. Some melt stubbornly high, others stay liquid in the fridge. This set of atoms, laid out as C9H17N2Br, pushes boundaries in solvent innovation. It offers new ways to separate chemicals without relying on often-toxic organics. That doesn’t mean the ride’s smooth. Some questions don’t quit: should we worry about persistence in the environment, or human safety if these liquids leak out of industrial tanks?
What’s Next?
Solving problems with ionic liquids starts with basics—structure and formula. From there, open reporting and honest sharing of results build trust. Research communities push for data on toxicity, biodegradability, and lifecycle impacts. In my experience, the labs ahead look brighter when everyone leans into transparency, not just patents or productivity metrics. C9H17N2Br doesn’t just fuel chemical cycles; it kickstarts honest conversations about the future of chemistry, energy, and sustainable tech.
What We’re Dealing With
1-Butyl-2,3-Dimethylimidazolium Bromide falls into the category of ionic liquids. These chemicals pop up in laboratories all over the globe. They’re favored for their low volatility and potential as greener solvents compared to many traditional organic materials. But just because something is “greener” or less volatile doesn’t make it harmless on the bench.
Direct Experience in the Lab
A few years ago, my lab tested a handful of imidazolium-based ionic liquids, including this one. No strong fumes burned my nose, and the liquid felt oily between gloves, not unlike mineral oil. Safety officers pointed out the importance of goggles, gloves, and a snug lab coat. Nobody wanted to treat this stuff like hand soap. One spill was enough—my billowing glove took the brunt of it, but lab protocol meant a trip to the sink, scrubbing with detergent, and tossing the gloves in the hazardous waste.
Safety by the Data
Toxicological data for 1-Butyl-2,3-Dimethylimidazolium Bromide isn’t as robust as, say, acetone or hydrochloric acid. What we know: ionic liquids in this family sometimes irritate skin and eyes, and they can be toxic if swallowed or inhaled in large amounts. The European Chemicals Agency pegs this compound as a skin and eye irritant. Peer-reviewed studies have flagged some imidazolium salts for moderate aquatic toxicity, as well as potential cellular toxicity at higher concentrations or after prolonged exposure.
The Risk in Practice
Handling safety rarely boils down to just reading the hazard label. Most accidents come from taking routine for granted. With 1-Butyl-2,3-Dimethylimidazolium Bromide, accidental splashes and absent-minded glove removal pose a bigger risk than acute poisoning. If you touch your face, food, or phone with contaminated hands—even without a burning sensation—you could end up with a rash or eye redness. Down the drain, waste mistreatment could cause trouble for aquatic life. Just because you don’t smell or feel immediate danger doesn’t mean you dodged one.
Supporting Facts
Researchers from the University of York flagged these ionic liquids’ persistence in the environment. Water treatment facilities don’t break them down easily. Regulators like OSHA haven’t published occupational exposure limits, mostly because these aren’t used outside of specialized settings. Some animal tests suggest possible reproductive effects at high doses, though this remains unclear.
Solutions and Responsible Handling
Treat every new bottle of 1-Butyl-2,3-Dimethylimidazolium Bromide with a healthy skepticism. Suit up—gloves and goggles aren’t overkill. Bench work needs trays or absorbent pads to catch spills. Keep the material away from drains. Waste collection protocols in universities almost always flag ionic liquids for separate disposal. Never pipette by mouth or work without ventilation, as powders and vapors sometimes surprise you.
Sharing knowledge is as valuable as the right gloves. Walk new lab members through hands-on handling before letting them work alone. If a spill happens, clear it up with dedicated absorbents and discard them the correct way. No one regrets playing it safe.
Modern chemistry keeps finding new uses for ionic liquids like this one. Safety keeps us running experiments another day, and nothing about 1-Butyl-2,3-Dimethylimidazolium Bromide changes that. Gloves, waste bins, and honest risk assessment trump personal bravado every single time.
Safety Starts at the Shelf
A compound like 1-butyl-2,3-dimethylimidazolium bromide doesn’t look dangerous at a glance, but treating it with respect saves a lot of trouble. From experience working in both academic and industry labs, the handling of ionic liquids never leaves room for casual habits. The problem with this one isn’t just its chemical nature. If mishandled, degradation and contamination quickly follow, and those headaches spiral into bigger safety risks and lost research time.
Watch Out for Water and Light
Here’s the thing about compounds based on imidazolium: they draw in moisture from the air like magnets. Leave the lid off, and next thing you know, your sample is swimming in humidity. That means storing 1-butyl-2,3-dimethylimidazolium bromide in a tightly sealed container isn’t just tradition, it’s necessary. Glass containers with screw caps, and a squeeze of Parafilm for good measure, give you a solid first line of defense. Polypropylene bottles work for bigger batches, but glass always gives a clear signal if something’s gone wrong.
Chemists often put this compound in a desiccator with silica gel or another effective drying agent. In my first research position, nothing killed sample quality faster than letting it sit anywhere humid. Even a few hours’ exposure can throw off the water content, changing how it handles in your reactions. Humidity also boosts the risk of slow decomposition, and nobody wants to see yellowing or strange crystals developing inside their jar.
Cool and Dark Is the Rule
Postdocs and old timers will agree: keep it as cool as you can without freezing. A room-temperature bench won’t ruin a single sample, but long-term storage near hot lights or sunny windows isn’t smart. Standard practice puts bottles in a chemical fridge, away from food and drink—segregation keeps everyone safe and the product pure.
The story doesn’t finish there. Even common fluorescent bulbs introduce enough energy to coax slow changes in some ionic liquids. While the material handles a lot before it breaks down, light-exposed bottles have a way of getting sticky over time. Wrapping containers in foil or using amber glass bottles blocks out most stray light, preventing degradation that turns samples useless.
Label Everything, Limit Air Exposure
A lot of chemists skip this step once or twice, until unlabeled clear bottles pile up at the back of the shelf. Labeling the name, synthesis date, and responsible person, then writing down each withdrawal in a logbook, lets you spot a problem before it snowballs. Open the bottle only in a dry atmosphere—ideally under an inert gas if you’re in a bigger lab with glovebox access. Nitrogen-purging after each use limits slow oxidation, which quietly sneaks up on poorly stored organics.
Solutions for Real Labs
Most chemistry teams streamline their storage plans. Regular checks every week discourage lazy habits. Labs train everyone to avoid returning unused powder to the main container—a source of cross-contamination that’s bitten more than one graduate student. Spill kits close by, dated inventory systems, and a common-sense rule to replace anything that looks “off”—it all helps keep the work reliable.
Proper attention to storage doesn’t eat much time, but the payoff is huge: reliable results, longer shelf life, and fewer safety scares. In my time with process development, the quiet discipline of proper storage meant fewer headaches and safer work. 1-butyl-2,3-dimethylimidazolium bromide may not be flashy, but the right storage sets the stage for all the discoveries to come.
A Closer Look at an Ionic Liquid
Working in chemistry labs over the years, I have seen a range of ionic liquids show up in research and industrial settings. 1-Butyl-2,3-Dimethylimidazolium Bromide stands out because it challenges the belief that all salts must be solid crystals at room temperature. Instead, this compound often shows up as a nearly colorless to pale yellow, viscous liquid. People who have handled it know the surprise that comes from pouring a “salt” when you’re used to pouring water.
Melting Point and Appearance
At room temperature, 1-Butyl-2,3-Dimethylimidazolium Bromide holds its liquid form, with a melting point generally recorded around 60°C. In my experience, if your lab bench sits in a chilly corner, this liquid creeps toward a thick, almost sticky consistency. This ability to avoid crystallization at lower temperatures proves useful in practical settings because nobody wants a solvent turning solid in the middle of an experiment. The clear, sometimes faintly yellow appearance tells you the synthesis worked, and you haven’t picked up random impurities from your glassware.
Viscosity and Solubility
The viscosity of 1-Butyl-2,3-Dimethylimidazolium Bromide tends to surprise those expecting something as thin as ethanol or acetone. This substance pours slowly, behaving almost syrup-like, reminding me of times I’ve accidentally spilled it and spent ten minutes cleaning my gloves. It’s not just the thickness that matters. Solubility offers another story. It dissolves readily in water, and has decent compatibility with polar organic solvents like methanol. This solubility matters for chemical processes because it opens the door to use as a versatile solvent or as a phase-transfer catalyst.
Hygroscopic Nature and Handling
Experience teaches you to store this ionic liquid with care. As a hygroscopic material, it absorbs water quickly from the air. In humid environments, if you leave the cap off too long, you can see it pulling in moisture, which changes its properties and can mess up experiments relying on a “pure” sample. This trait always reminds me how crucial tight containers and desiccators become in everyday lab work.
Thermal Stability and Conductivity
With a decomposition temperature above 200°C, 1-Butyl-2,3-Dimethylimidazolium Bromide handles moderate heating without breaking down. I recall several times when a reaction mixture started climbing higher on the thermometer, but this ionic liquid stood firm, resisting degradation. The thermal stability serves labs well, letting reactions run at elevated temperatures without turning the whole flask into a mess of decomposition products. Another factor to consider is its ionic conductivity. The charged nature of the molecule, combined with the mobility of its ions in liquid form, leads to decent electrical conductivity compared to many traditional organic solvents.
Environmental and Practical Impact
Unlike classic organic solvents, this ionic liquid produces almost no vapor pressure. This means less inhalation risk and lower environmental impact from air emissions. I remember labs switching to ionic liquids like this for specific reactions just to reduce odors and hazard levels. The push for greener chemistry moves products like 1-Butyl-2,3-Dimethylimidazolium Bromide closer to the center of attention.
Moving Forward
For chemists wanting to swap out volatile organic solvents for something with less environmental downside, understanding the details of this ionic liquid matters. Reliable storage, good ventilation, and keeping track of water absorption all help avoid headaches and mistakes. Industry uptake will rely on how easily people adapt to its physical quirks, but the possibilities look promising for those ready to experiment with something different.