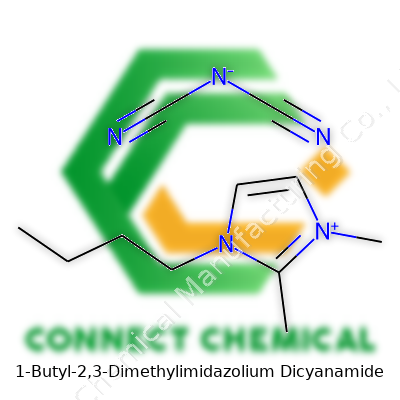
1-Butyl-2,3-Dimethylimidazolium Dicyanamide: An In-Depth Commentary
Historical Development
The search for safer and more efficient solvents led many chemists to investigate imidazolium-based ionic liquids during the late 20th century. 1-Butyl-2,3-dimethylimidazolium dicyanamide started gaining traction once researchers noticed the unique mix of low toxicity and solid stability among similar compounds. In academic labs and in the pages of journals, it quickly became clear that this ionic liquid offered more than novelty; chemists saw real potential for green chemistry practices. I recall conversations with colleagues who switched from volatile organic solvents to ionic liquids, finding the learning curve manageable compared to difficult solvent-replacement projects in the past. Moments like those paved the way for more sustainable chemistry, and this particular ionic liquid emerged from a blend of practical demand and innovative synthesis work.
Product Overview
1-Butyl-2,3-dimethylimidazolium dicyanamide stands apart from older solvents in how it handles demanding lab routines. Instead of evaporating or breaking down under moderate heat, it stays put while remaining easy to work with. The cation, based on butyl-imidazolium, pairs with the dicyanamide anion to form a homogeneous liquid at room temperature. Industry uses it in both small batches and pilot-scale runs, not just as a chemical curiosity. The product’s low vapor pressure and high solubility with many substances keep it attractive for academic research and potential commercial uses. Over time, adoption grew where people wanted a solvent that both dissolved many materials and could support electrical applications, and its reputation followed that success.
Physical & Chemical Properties
Pour this liquid into a beaker and you’ll see a colorless, nearly odorless substance that feels somewhat viscous. On the chemical side, it forms a stable ionic pair, and those dicyanamide anions contribute to notable thermal resilience. With a melting point usually well below 25°C, it flows steadily in most lab spaces. Its polarity, paired with low volatility, means it sticks around during heating, and survives even extended reactions. Chemists have found that it resists hydrolysis and won’t degrade if a little water sneaks into an experimental setup. Its density falls close to that of water but carries just enough mass to make handling feel distinct from lightweight organic solvents. Electrical conductivity proves strong enough for electrochemical uses, rivaling other ionic liquids of its generation.
Technical Specifications & Labeling
Manufacturers list purity ranges above 97% for research-grade stock. Detailed batch labels also outline water content, which usually stays under 1% by weight—this matters in reactions where every drop counts. Density sits near 1.07 g/cm³, and reliable documentation includes refractive index and conductivity measurements. Suppliers tag each bottle with CAS numbers and hazard codes, a habit that keeps facilities compliant and research reproducible. Labels warn against direct skin and eye contact, and provide clear instructions for storage—cool, dry places with secure lids recommended. From my experience, precise tracking of batch numbers and expiration dates avoids headaches down the road, especially when reordering for long projects.
Preparation Method
Synthesis often starts from the base imidazole ring, which reacts in sequenced steps to attach butyl and methyl groups at the right spots. Quaternization with butyl halide builds out the cation, and careful control prevents excess byproducts. To generate the dicyanamide salt, the halide exchanges with sodium dicyanamide, producing the ionic liquid and a sodium salt byproduct. Filtration and careful vacuum drying remove water and trace organics. Rigor during each step results in cleaner product and higher yields, which often make the difference between a shelf-stable solvent and one that breaks down before its time.
Chemical Reactions & Modifications
Adding functional groups to the imidazolium core or tweaking the alkyl chain lets chemists fine-tune properties for a specific task. The dicyanamide anion stays reactive toward certain transition metals, enabling coordination chemistry that broadens application possibilities. Chemists in materials science often modify this liquid for new polymers or to stabilize nanoparticles. Small tweaks to the cation alter polarity, melting point, or solubility, and each adjustment opens new windows in catalysis, separation, or electrochemical systems. In hands-on work, the ability to customize the ionic liquid instead of switching solvents altogether creates room for innovation without sacrificing routine or safety.
Synonyms & Product Names
This compound goes by several names across publications and supplier catalogs. Most chemists recognize the abbreviation [BMMIm][DCA], but suppliers might list n-butyl-2,3-dimethylimidazolium dicyanamide or use “ionic liquid” as a catch-all. CAS numbers (such as 79922-29-7) anchor these labels, keeping cross-referencing straightforward. Remembering alternate names or identifiers helps when digging through literature or placing orders from different vendors. Even small labs benefit from keeping track of synonyms to avoid duplication or mix-ups.
Safety & Operational Standards
Lab workers find that 1-butyl-2,3-dimethylimidazolium dicyanamide needs careful handling, though fewer safety alarms ring compared to volatile aromatics. Skin and eye contact bring irritation risks, and ingesting or inhaling vapor call for medical consultation. Fume hoods stay vital during heating, and spill containment means absorbent materials and routine wipe-downs with appropriate cleaners. Standard operating procedures include double-checking glove compatibility, as some nitrile types soften after prolonged exposure. Waste accumulation needs labeling and tracking, following policies for organonitrogen compounds. Safety training for new technicians includes clear demonstrations, since accidents usually happen when teams skip these small but crucial steps.
Application Area
Research labs prize this ionic liquid for its solvating power, especially in organic synthesis and catalysis. Electrochemists work with it as a medium for redox reactions, taking advantage of its high ionic conductivity and wide electrochemical window. As a solvent for cellulose, it opens doors in biomass processing where traditional solvents fail. Analytical chemists use it for sample preparation and separation, particularly when selecting media for high-performance liquid chromatography. Battery developers examine it for non-flammable electrolytes, aiming for safer energy storage. Expansion in 3D printing, polymer synthesis, and nanomaterial stabilization demonstrates where this compound fits in cutting-edge technology. Every new application pushes boundaries, backed by field reports and peer-reviewed studies, while industry interest supports the translation from lab scale to pilot runs.
Research & Development
Development teams and academic groups tackle hurdles like thermal degradation, long-term stability with metals, and compatibility with a growing list of substrates. I’ve seen research projects pivot once a compound like this enters the mix, unlocking workarounds for formerly intractable synthesis bottlenecks. Collaboration with engineers encourages new reactor designs and automation strategies, aimed at scaling up ionic liquid-mediated reactions. Every successful demonstration brings closer a vision of sustainable chemical manufacturing—not as a buzzword, but as built-in practice. Researchers continually test alternate anions and new cation structures, sometimes to squeeze out a few percent more yield, or to cut waste in recyclability studies. These incremental advances add up, and expert teams share findings in conferences and journals, feeding a cycle where industry prototypes become tomorrow’s standard process.
Toxicity Research
Understanding toxicity matters for any substance destined to leave the lab bench. Peer-reviewed work shows this ionic liquid lands between older organic solvents and the safest, water-like alternatives. Acute toxicity remains low, but researchers examine chronic effects and any risk from cumulative exposure, especially as more gets used outside laboratory settings. Environmental scientists monitor breakdown products after disposal, mapping out pathways that could affect soil or groundwater. Every few years, updated toxicity reports prompt manufacturers to review data sheets or recommend extra safeguards. This cycle of scrutiny protects both workers and communities, a process built on real-world testing and transparent reporting.
Future Prospects
Advances in green chemistry and sustainable production will boost the profile of ionic liquids such as 1-butyl-2,3-dimethylimidazolium dicyanamide. As industries work harder to meet stricter environmental benchmarks, this compound presents real answers—as a tool for less hazardous processing, and as a piece of the puzzle in battery technology or biorefineries. Ongoing research may uncover modifications that further lower toxicity or ramp up efficiency for single-use manufacturing. I see a path where tailored versions of this ionic liquid show up in medical devices or advanced coatings, once hurdles in safety and cost come down. Universities and companies, staying focused on both practical application and rigorous testing, will shape what comes next, turning today’s specialty solvents into building blocks for the circular economy.
Stepping Into the World of Ionic Liquids
Not many people outside chemistry circles talk about a substance called 1-butyl-2,3-dimethylimidazolium dicyanamide, but this mouthful does some serious work behind the scenes. In the lab, the science world sees this compound as a rising star among ionic liquids. These designer liquids stay liquid at low temperatures, don’t evaporate like water or gasoline, and handle heat and cold with strong staying power. That gives them an edge over older, volatile chemicals, which carry health risks and environmental headaches.
Opening the Door to Better Solvents
Solvents play a huge role in making medicines, electronics, and plastics. Traditional solvents like toluene and acetone evaporate, polluting indoor air and adding fuel to wildfires, explosions, or chronic headaches. More careful handling slows projects and racks up waste. 1-butyl-2,3-dimethylimidazolium dicyanamide sidesteps those problems. Its non-volatile nature lets labs and factories cut down on air pollution and fire risk. It dissolves a wide mix of materials, from metals to complex organic molecules, and the product often needs less cleaning at the end of the process. That means less waste to haul away, less energy spent on purifying, and a clearer path towards greener manufacturing.
Changing the Game in Battery and Electronics Manufacturing
Anyone following advances in batteries and supercapacitors knows scientists are on the hunt for safer and more efficient electrolytes. The dicyanamide part brings electrical stability, meaning it won’t break down and corrode sensitive materials at high voltage. Makers use this ionic liquid when putting together next-generation batteries and screens for devices that can last longer and get slimmer. I’ve read research showing batteries built with these liquids lose capacity more slowly over hundreds of charge cycles, raising hope for less electronic waste in landfills.
Greener Catalysis and Synthesis
Making pharmaceuticals, clean fuels, or specialty chemicals usually requires high pressure, heat, and nasty catalysts. With 1-butyl-2,3-dimethylimidazolium dicyanamide, you can toss away some of the most toxic or high-energy processes. Reactions run at lower temperatures. Chemists have pointed out to me you can recycle the liquid at the end just by washing it or turning up the heat. I worked in a research setting that started swapping in ionic liquids, including this one, just to track worker safety improvements. Complaints about fumes and headaches quickly went down, and industrial accidents dropped, too.
Sustainability and Looking Forward
The world of ionic liquids still has some homework to finish. Regulatory agencies keep a close watch on toxicity and breakdown products, since some of these molecules could stick around in soil or water if not handled with care. Engineers and chemists need trustworthy data to understand the real risks and chart the safest course. Still, options like 1-butyl-2,3-dimethylimidazolium dicyanamide already help companies shrink their environmental impact compared to old-school organic solvents.
How Scientists and Factories Put It to Use
This molecule shows up in chemical plants, batteries, and research labs all over the world. I’ve talked to people in energy research who see ionic liquids as essential for tomorrow’s fuel cells and power grids. Trainers in lab safety bring up how these new solvents let students try advanced techniques without needing a face mask or fume hood at all times. With careful handling and more research, 1-butyl-2,3-dimethylimidazolium dicyanamide stands out as the kind of chemical that could make the science world more sustainable, practical, and safer for everyone.
Navigating the Safety Questions Around Modern Ionic Liquids
The lab bench and the factory floor keep getting stocked with new chemical tools, and 1-Butyl-2,3-Dimethylimidazolium Dicyanamide isn’t some obscure compound only known to a handful of chemists. Folks in academic research and specialty industries face questions about its use and, of course, its risks. The rise of ionic liquids has promised greener chemistry for tough jobs, but the phrase “greener” sometimes hides hazards that hit less obvious.
Anyone who has worked with solvents knows organic chemistry is a story of trade-offs. For a while, people got excited about ionic liquids like this one, because they don’t evaporate easily. Fewer fumes look like a gift—until you start reading into toxicity data and human exposure cases. There’s usually a catch hiding somewhere in those Safety Data Sheets.
Looking at 1-Butyl-2,3-Dimethylimidazolium Dicyanamide, its very structure signals the need for care. Dicyanamide contains cyano groups. Cyanide doesn’t play nice in the body, and while dicyanamide is not straight cyanide, parallels cannot be dismissed. The imidazolium cation family has some interesting biological action. Studies show cytotoxic effects in cell cultures, especially with prolonged exposure. Skin irritation and eye damage get reported in lab settings, even when liquid didn’t get splashed directly—sometimes, you only realize something’s off when someone’s hands have tingled for hours after cleaning a spill.
I’ve watched researchers downplay concerns by calling it “new and green,” but a responsible chemist checks deeper before dismissing risk. The European Chemicals Agency posts data that underline toxicity to aquatic life—something as stubborn as an ionic liquid is going to hang around in water and soil. What goes down the drain has a way of showing up somewhere it shouldn’t be. Those involved in industrial use have recorded breathing in fumes if they heat or spray these compounds. Material safety guidelines in Korea and the US echo those warnings. Direct handling is not something done bare-handed, no matter how safe a product label reads.
Safe handling starts with common-sense protection: gloves, safety glasses, and strong ventilation. Closed systems work best for transfers. Spills gum up benches and can lead to awkward conversations with your EHS officer. Disposal doesn't mean just hosing down a drain, since water authorities do not take kindly to cyanide-related compounds. Waste collection routes make a difference, too—if you’re pushing for green chemistry, that needs to include waste management, not just reaction steps.
Education and transparency rank just as high as gloves and goggles. I’ve seen more than one new chemist try to cut corners, thinking a fancy chemical name means less risk. Companies should run regular in-person training and not just rely on digital modules. Lab culture tends to pass practices down silently, so senior researchers should step in and talk through real-life scenarios—what to do if your glove rips, where the eyewash actually is, why you shouldn’t microwave your lunch near the hoods. Human error outpaces guidelines, so never assume anyone “just knows” because they’ve read a label before.
As much as we need better, more efficient chemicals, the basics never change. Handle 1-Butyl-2,3-Dimethylimidazolium Dicyanamide with the same respect you’d give any strong solvent or reagent. Ask questions. Read updated reports. Share best practices in meetings, not just in paperwork. Chemical safety still works best as a team sport—and honest conversations keep everyone safer.
Keeping Chemical Integrity Top of Mind
Some chemicals handle rough spots better than others, but 1-Butyl-2,3-dimethylimidazolium dicyanamide calls for careful attention. Years working around specialty chemicals have shown me that storing ionic liquids like this one often gets overlooked, especially outside the world of academic labs and regulated plants. Still, missing a few details when finding it a shelf can turn a harmless-looking sample into a worksite hazard.
Why Storage Choices Matter
Chemically, 1-Butyl-2,3-dimethylimidazolium dicyanamide belongs to a family of ionic liquids prized for their thermal stability, low volatility, and utility in synthesis and catalysis. The dicyanamide anion brings its own quirks — strong reactivity with acids, and a tendency to break down if exposed to moisture or intense heat over time.
Practical experience tells me that trying to save time or resources with short-cuts degrades product quality and intercepts safety. For instance, sample vials left uncapped or stored on open shelves start to pick up ambient moisture quickly, clumping or solidifying. Loss of purity creeps up and possible toxin formation becomes much more likely.
Core Storage Guidelines
Environment shapes chemical behavior. For this salt, clean, dry places always help keep problems away. I learned early on — ambient humidity is the main culprit behind sample spoilage. Store in tightly sealed, chemical-resistant containers; borosilicate glass vials with threaded caps work well. Parafilm cuts down leakage, but don’t rely on it as a long-term stopper.
Temperature puts its own spin on things. Though ionic liquids like this handle moderate heat, I set temperature limits below 30°C to ward off slow decomposition. Standard lab refrigerators, set just above freezing, usually work — but don’t freeze it unless the product sheet clearly endorses deep cold. Rapid solidification creates crystals, which can compromise even a new batch.
Protecting from Light and Contaminants
Sunlight and harsh UV can prompt chemical changes. Opaque bottles beat clear glass, especially if the chemical sits out for weeks. If supplies run low, transfer anything left in small aliquots to reduce air exposure with each use.
Over time, fine contaminants sneak into open samples. Use disposable pipettes for withdrawal; clean tweezers and gloves prevent cross-contamination. From biotech plants to university teaching labs, I’ve seen how reusing spatulas leads to unexpected reactions and sample clouding.
Addressing Hazards and Building Good Habits
Labeling saves lives and budgets. Each time I get a new batch, I relabel the bottle with date received, date opened, and expiration date if known. Sheet records back up these labels. Storing away from acids, bases, or oxidizers lowers the odds of exothermic mishaps — a lesson hammered home by more than one emergency drill.
Waste needs its own attention. Neutralize any residue using recommended protocols, and place old material in hazard bins, not down the drain. Safety Data Sheets from certified sources often provide extra details tailored to institutional requirements.
Better Storage: Shared Experience and Future Advice
From my own projects, I know that good storage is more habit than science. Care consistently applied — dry hands, labeled vials, tight caps, cool rooms — stands between you and wasted inventory, preventable incidents, or failed experiments. Chemical safety officers often say, “People forget the basics.” Those basics are what keep the lab running for everyone who comes after.
Digging Into the Structure
Chemicals like 1-Butyl-2,3-dimethylimidazolium dicyanamide may sound intimidating, yet breaking down their structure gives real insight into how they shape new technology. The core piece here, the imidazolium ring, comes from two nitrogen atoms positioned across a five-membered ring, flanked by three carbon atoms. Add two methyl groups at positions 2 and 3, plus a butyl group attached to the first carbon, and the molecule picks up extra bulk and stability. The result: a cation that resists breaking down.
The dicyanamide part, the anion, shows up with two cyano groups—each a carbon dimly bonded to a nitrogen—straddling a nitrogen. This trio forms an anion that spreads its charge across those nitrile groups, making it a strong partner for the imidazolium cation. The formula, to get technical, shows up as C11H18N5 for the total compound.
Why Its Shape Matters Outside the Lab
A researcher running extractions or working in electrochemistry wants an ionic liquid that won’t evaporate away or corrode equipment. This specific chemical—the cation with flexible butyl and methyl tails, and the dicyanamide’s smooth electronic surface—brings a suite of practical features. It doesn’t flash off at room temperature, barely mixes with water or many solvents, and easily dissolves plenty of organic compounds, especially ones that give solvents trouble.
Think about batteries. Lithium-ion makers hunt for stable conductors that boost longevity and keep cells cooler. Ionic liquids like this one help limit flame risk—unlike some volatile electrolytes. They keep their shape, so the electronics inside vessels and cells remain consistent. Scientists need this kind of chemical stability for reliable devices. Sitting in the lab, one sees how a new salt on a battery prototype can mean a breakthrough in safety.
Looking at Environmental Impact and Solutions
There’s curiosity—and concern—around ionic liquids. They don’t evaporate, so indoor air stays cleaner, but most still challenge ecosystems if spilled. Traditional organic solvents bring headaches from vapors and disposal, yet ionic liquids don’t just vanish when poured down a drain. Here’s where careful choices count. Making salts like 1-butyl-2,3-dimethylimidazolium dicyanamide from well-chosen feedstocks, developing better recovery procedures, and looking at end-of-life for these salts avoids environmental buildup. The good news: teams across the world now set up recycling loops and push for safer synthesis routes.
For someone who handled solvents during graduate research, the jump to ionic liquids meant fewer headaches and less health worry. Regulations now push for safer chemistry—green chemistry principles recommend considering toxicity, minimization of waste, and simple separation. As labs push forward, these choices pay off for workers, communities, and water sources nearby.
Unlocking More Potential
Looking past basic structure, the design of 1-butyl-2,3-dimethylimidazolium dicyanamide hints at an evolving landscape where molecular tweaks unlock new roles. Fine-tuning size and arrangement of side groups means improving solubility or flexibility for pharmaceutical processes, or turning ionic liquids into carbon capture agents. Students and industry workers now have a playground of safe, effective solvents to pick from, as understanding deepens around structure-function links. Skipping the mystery, the focus now moves to matching molecular design with real-world targets: cleaner manufacturing, safer workplaces, and creative new applications.
Why Chemicals Like This Matter
Years spent around labs and manufacturers taught me that not every chemical with a long name spells trouble, but you’d better know what you’re dealing with. 1-Butyl-2,3-Dimethylimidazolium Dicyanamide fits squarely into that category. Folks seeking this ionic liquid probably work in niche research or production fields. Maybe folks spotted an intriguing paper on greener solvents and now want to replicate results or source small batches for specialized work.
Let’s be straight: this isn’t paint thinner from the corner shop. You need clear purpose and real knowledge. Its applications—whether in battery studies, catalysis, or solvents—make it an interesting tool. At the same time, regulations and safety track pretty close to any substance with cyanide groups in its structure.
Legitimate Sourcing Beats Cut Corners
I once thought buying specialty chemicals was as simple as finding one of those huge online platforms: punch in the name, click, and wait for the doorbell. That’s rarely how it plays out. Most sellers will ask for business details, intended use, and credentials—especially for compounds with toxicity risks. I've watched colleagues get frustrated with layers of screening, but all it takes is one incident for everyone to appreciate the checkpoints.
For research quantities, well-known suppliers like Sigma-Aldrich, Alfa Aesar, and TCI America usually stock niche compounds like this. You’ll need to register an institutional account and supply detailed project info. Safety Data Sheets (SDS) will spell out hazards and handling steps, so be prepared to show compliance with your lab’s standards. Sometimes new researchers try Amazon or Ebay, but listings there rarely measure up in traceability or legal compliance, especially for regulated substances. Reading forums reveals more than a few stories about seized shipments or outright scams.
Watching Out for Legal and Safety Issues
My time working with industrial procurement taught me a few hard lessons. Chemicals containing dicyanamide can draw regulatory scrutiny in many places, including Europe and the United States. Laws shift, but one fact stays the same: authorities want to prevent misuse. Shipping, storage, and disposal rules bite hard. Always ask suppliers to confirm current status and never gloss over requirements for import permits or end-use declarations. Colleges and companies usually need to involve their Environmental Health and Safety office before even placing an order.
Some folks think "small quantity, no big deal." The opposite holds true, based on years seeing what even a few grams can do in the wrong setting. Anyone handling substances like this must have chemical-resistant gloves, goggles, and a proper fume hood. Accidental contact or poor storage can mean immediate health problems and violations. Mistakes end careers; playing safe protects everyone.
Balancing Research Freedom With Common Sense
It feels tempting to look for fast shortcuts if project deadlines loom or samples run low, but shortcuts with regulated chemicals rarely pay off. Real science and good business build on solid sourcing, traceable paperwork, and open communication with safety staff. Collaborating with established suppliers and respecting safety requirements saves you more hassle in the long run. In my own work, that approach built credibility and avoided costly errors. Take time to source 1-Butyl-2,3-Dimethylimidazolium Dicyanamide through the right channels—it’s a sign of professionalism you won’t regret.