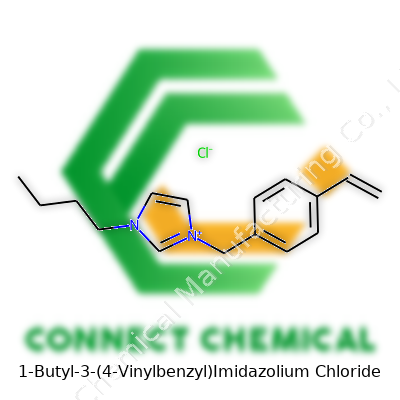
1-Butyl-3-(4-Vinylbenzyl)Imidazolium Chloride: An In-Depth Commentary
Historical Development
Talk with any chemist who has explored ionic liquids, and you’ll hear stories about those early days when researchers were cracking open doors to green chemistry. 1-Butyl-3-(4-vinylbenzyl)imidazolium chloride didn’t just show up out of nowhere. The history of its design goes back to the surge of interest in imidazolium salts. Chemists working in the late twentieth and early twenty-first centuries understood that imidazolium cations allowed for remarkable tunability regarding melting points, solubility, and thermal stability – valuable in multiple scientific fields. Through incremental discoveries, researchers moved from simple alkylated imidazolium compounds to more specialized derivatives, bringing in functionalized groups such as 4-vinylbenzyl to expand applications—especially in polymer and catalysis research. Each step in its evolution reflects distinct changes in laboratory focus: from solvation to polymeric materials, to smart solvents for new reaction types.
Product Overview
1-Butyl-3-(4-vinylbenzyl)imidazolium chloride represents a class of ionic liquids with a foot on both polymer chemistry and catalysis. By combining a butyl group and a vinyl-substituted benzyl on the imidazolium core, this compound meshes the strengths of ionic mobility with a handy vinyl group for polymer crosslinking. In the right hands, this product streamlines the creation of functional materials, opening up routes unavailable with legacy ionic liquids. Its use isn't just a theoretical exercise; researchers actually bring this salt onto the lab bench for ion-conducting membrane prep, molecular imprinting, and tailored material synthesis.
Physical & Chemical Properties
This salt exhibits a solid or highly viscous liquid form at room temperature, depending on purity and local humidity. The imidazolium ring and chloride counterion grant water solubility, but you’ll spot some hydrophobic tendencies thanks to the butyl and vinylbenzyl groups. Its melting point falls in the range typical for quaternized imidazolium salts—often reported between 80°C and 120°C depending on sample handling. Chemists respect the vinyl group for its double bond, ready to undergo copolymerization. At the same time, its chemical structure imparts significant electrochemical stability, allowing for work in higher-voltage environments. This property pushes the salt into experimental battery electrolyte compositions. In my own time at the bench, solutions featuring this compound never failed to surprise – it dissolves well in water and polar organic solvents, making it incredibly adaptable.
Technical Specifications & Labeling
Suppliers usually deliver 1-butyl-3-(4-vinylbenzyl)imidazolium chloride as a white to off-white powder, sometimes appearing as a waxy solid if stored improperly. Purity typically hits 97% or better, verified by NMR and ion chromatography. Labels cover not only CAS and EC numbers, but also potential hazard warnings. Standard containers block UV light, a nod to the vinyl group’s sensitivity. Chemically, the labels detail the empirical formula (C16H21ClN2) and molecular weight (276.81 g/mol), important for bench calculations. Anyone ordering from reputable vendors should check batch certificates for traces of hydrolysis or polymerization, as those lower quality and performance.
Preparation Method
Preparation requires real attention to detail. Lab teams usually start with 1-butylimidazole and a suitable 4-vinylbenzyl chloride derivative. Slow, controlled alkylation in an inert atmosphere helps to prevent premature polymerization of the vinyl group—a trap for the hasty. Maintaining anhydrous conditions remains key, as water content leads to unwanted hydrolysis side-products. After completion, the mixture undergoes careful filtration and solvent washings to pull out unreacted starting materials. Full drying over vacuum helps guarantee a pure, stable ionic liquid. I recall troubleshooting this step, where even a trace of unreacted vinylbenzyl chloride contaminated polymerization outcomes downstream.
Chemical Reactions & Modifications
What distinguishes 1-butyl-3-(4-vinylbenzyl)imidazolium chloride lies in the vinyl handle. Synthetic chemists covet this group for copolymerization, often blending it with acrylates or styrene to build ion-conductive networks. The compound can anchor to solid supports by free-radical or anionic polymerization—handy for molecular imprinting applications or recycling supports. The chloride anion frequently undergoes metathesis, allowing swaps with PF6- or BF4- to enhance hydrophobicity and tune ionic conductivity. This flexibility means chemists can adjust the compound for ionic membranes or gel electrolyte systems without reinventing their entire workflow. Over time, labs who started with this chloride salt soon built whole families of related ionic liquids and copolymers.
Synonyms & Product Names
Depending on who you ask or which catalog you read, 1-butyl-3-(4-vinylbenzyl)imidazolium chloride goes by several names. Some vendors shorten it to “BVBICl” or, less commonly, “VBImCl.” Its systematic nomenclature often appears in patents and research papers, cementing clarity for international readers. Those in the field develop a shorthand, knowing each abbreviation carries the legacy of several years of synthesis and benchmarking. For researchers working across languages and disciplines, recognition of these names speeds up literature searches and order fulfillment, avoiding confusion with other imidazolium-based salts.
Safety & Operational Standards
Laboratory teams handling this compound keep one eye on safety data sheets and another on personal protective equipment. 1-butyl-3-(4-vinylbenzyl)imidazolium chloride counts as an irritant, especially with long skin or eye contact. Chloride salts, while manageable, trigger corrosion in certain metals, leading to strict handling protocols around steel equipment. Fume hoods serve as standard practice during synthesis and polymerization. The vinyl group’s reactivity also means chemists avoid direct sunlight and unnecessary heat, limiting the risk of runaway polymerization. Waste treatment procedures cover both chloride and imidazolium toxicity, reflecting institutional and government safety guidelines. Colleagues swapping best practices in the hallway often trade tips on container labeling or disposal, feeding into a safer research culture.
Application Area
This ionic liquid finds a home in laboratories focused on polyelectrolyte membranes, especially in next-generation fuel cells and batteries. The vinyl group locks into place via polymerization, yielding stable, conductive materials for sensors or energy applications. In some cases, this salt acts as a monomer in ionic polymer matrices, delivering fixed-site charge carriers required for proton exchange applications. Analytical chemists have explored its potential as a stationary phase modifier, boosting selectivity in separation science. Environmental engineers run experiments with it in pollutant extraction, leveraging tunable solubility and electrostatic interactions. From conversations with researchers in the field, this compound consistently appears on the short list for anyone designing ion-exchange systems or targeted separation tools.
Research & Development
Imidazolium-based ionic liquids have pushed the boundaries of material science for the last couple of decades. Investigators keep broadening the scope for 1-butyl-3-(4-vinylbenzyl)imidazolium chloride, driving work in photopolymerization, electronic materials, and nanocomposites. Joint projects between academic chemists and industrial labs often test this compound’s limits by combining it with layered silicates or graphene, aiming to boost conductivity or create hybrid materials that resist fouling and degradation. Newer projects often focus on green chemistry metrics: finding ways to recycle or upcycle spent ionic liquid materials. In group meetings, teams share updates on chemical modifications, hoping to break new ground in sustainable materials and energy harvesting fields.
Toxicity Research
Safety remains front and center, both for workers and the broader environment. Early studies suggested imidazolium salts carry moderate aquatic toxicity, especially for low molecular weight variants; researchers test newer compounds, such as the vinylbenzyl derivative, for chronic effects. Bioaccumulation rarely bothers major environmental protocols due to low volatility and high aqueous solubility. Regulatory offices in Europe, the US, and East Asia have reviewed these compounds for use in industrial and academic settings, prompting stricter waste handling and suggestions for closed-loop water systems. The conversation in lab safety circles always comes back to minimizing worker exposure and preventing release into waterways—a challenge that technology and training both help address.
Future Prospects
Ongoing research hints at opportunities beyond what current applications provide. Polymeric ionic liquids anchored with vinylbenzyl groups stand poised for broader roles in water purification, biomedical material science, and flexible electronics. As sustainability standards rise, teams invest in greener synthesis methods, recycling protocols, and non-chlorinated alternatives. Big-picture thinkers in chemical engineering imagine uses in electrochemical carbon capture, low-temperature fuel cells, and smart coatings. Whenever new regulations or environmental constraints surface, the modular nature of the imidazolium core and vinyl group offers a path forward—letting researchers adapt or reengineer materials to keep pace with changing expectations and applications. Anyone following the field expects to see 1-butyl-3-(4-vinylbenzyl)imidazolium chloride at the heart of polymer science’s next wave.
Modern Chemistry in Day-to-Day Industry
Mentioning a name like 1-butyl-3-(4-vinylbenzyl)imidazolium chloride can sound intimidating. In practice, this compound holds a surprising amount of utility. Anyone with experience in material science or applied chemistry might notice how ionic liquids have been making waves, especially compounds built around imidazolium cores. These molecules match efficiency with creativity in a landscape that asks for stronger, nimbler, and more reliable building blocks.
Polymer Science and Smart Materials
This compound earns its stripes in the polymer sector. Through direct experience working with polymerization, it becomes clear that this imidazolium salt does more than fill a small niche. Scientists use it as an ionic liquid monomer, and the vinylbenzyl group lets it join up during polymer chains’ formation. This isn’t just academic—the method unlocks new ways to give plastics unique electronic, ionic, or thermal behaviors. In laboratories, professionals formulate membranes using compounds like this one to control ion or molecule movement. Fuel cells, for instance, rely on those unique properties to separate gases and conduct protons or ions. Performance jumps when these new polymers join the line-up, especially in applications where traditional plastics struggle.
Charge Transport in Batteries and Capacitors
Rechargeable batteries and supercapacitors constantly fight against inefficiency and capacity fade. Some folks I know in battery R&D turn to ionic liquids because they serve as safer, less volatile electrolytes. Here, 1-butyl-3-(4-vinylbenzyl)imidazolium chloride brings good chemical and electrochemical stability. Its structure helps design solid yet flexible electrolytes inside next-gen lithium batteries. There's a clear focus on safety, too—traditional electrolyte fluids burn easily, but these ionic liquids barely catch a spark. Engineers working with energy storage like the steady current and lower cycling losses they can get from these sections of the periodic table.
Helping Separate and Clean Waste Streams
Industrial plants create streams full of tough-to-remove compounds. Ionic liquids prove helpful for extracting valuable metals or removing toxic pollutants. For example, the task of pulling rare earth metals out of mining residues or removing heavy metals from wastewater gives 1-butyl-3-(4-vinylbenzyl)imidazolium chloride a real-world job. Growing interest in how imidazolium-based liquids latch onto specific ions has only increased. Sustainable chemistry means less solvent use, lower emissions, and the chance to recover valuable materials rather than just junking them.
Catalysis and Green Chemistry
Catalysts drive efficiency on factory floors and research benches alike. Chemists have shown, through direct trials, how ionic liquid catalysts made with this compound speed up chemical reactions that would otherwise lag. It’s about getting more product from less energy or fewer toxic inputs. For reactions that need precise control, using this chloride-based ionic liquid often pays off. It remains stable, doesn’t evaporate, and can sometimes be recycled back into another round of reactions. As pressure mounts to cut planet-warming emissions, using ionic liquids over traditional organic solvents opens a path to greener industrial methods.
Looking at Solutions and Challenges
New, specialty chemicals sometimes turn out expensive. The cost to produce imidazolium chloride variants can slow down adoption in big, price-sensitive markets. Open collaboration between research labs and manufacturers can address this, driving new breakthroughs in scalable synthesis. Life cycle analysis and thorough toxicity checks must also catch up to the pace of innovation—especially as these compounds leave the lab and enter widespread production.
The journey of 1-butyl-3-(4-vinylbenzyl)imidazolium chloride reflects a larger shift happening in chemistry—toward smarter, safer, and more adaptable materials, all while keeping real-world limitations and environmental costs in view.
The Backbone of Molecules: What Structure Really Says
A chemical structure says a lot about a compound. You can look at its shorthand formula and spot patterns, but the real story sits in how atoms line up and connect. Growing up with a shelf of old chemistry textbooks, I’d spend evenings drawing hexagons and lines, tracing caffeine, water, and benzene rings. Each one tells a story about how atoms share electrons—who’s holding hands with whom and who’s reaching for a third or fourth bond.
Take glucose, for instance. Its formula, C6H12O6, rolls off the tongue in science class, but focus on the structure and you see a six-membered carbon ring, with oxygen tucked into the mix. That exact geometry is why runners pound Gatorade and why plants stash energy as starch. Change a corner in that ring, and the compound acts differently—not just in the lab, but inside your body.
More Than Letters and Numbers: How Structure Shapes Use
Consider acetaminophen, a household name for headaches. The formula, C8H9NO2, looks unassuming. Peek at its structure and you’ll see an aromatic ring, an amide group, and a hydroxyl group. This arrangement lets it block pain signals in brains without causing ulcers like some anti-inflammatories. Pharmaceutical chemists have learned, through decades of trial and success, that molecular structure isn’t just academic—tweaks in arrangement help avoid nasty side effects and boost effectiveness.
Environmental impact ties directly to structure, too. PFAS—the so-called “forever chemicals”—draw concern partly due to their sturdy carbon-fluorine bonds. Those bonds won’t break down in sunlight or tackle microbes. The strength in structure locks PFAS into soil and water for generations, a clear sign that understanding chemical architecture helps us protect what matters.
Connecting Structure to Everyday Life
Chemistry isn’t locked in laboratories or behind goggles. Most people have cooked with table salt—sodium chloride, NaCl. Its structure forms a cubic lattice, which gives salt its crystal character and crunch. Even food flavor depends on arrangement; MSG (monosodium glutamate, C5H8NO4Na) unlocks taste receptors because of its unique shape.
My personal run-in with a chemical structure happened years ago in the garden. Vinegar, or acetic acid (CH3COOH), knocked out weeds better than soap, thanks to its simple but powerful arrangement. That two-carbon chain bonded to a carboxyl group leans acidic. It’s a humble molecule, but its structure gives it punch.
Solutions: Clarity, Responsibility, and Innovation
Precision in reporting chemical structures gives everyone—from researchers to regulators—a clear playbook. Misunderstanding a structure can leave gaps in safety data or drug information. Fact sheets and chemical databases should include not just formulas but diagrams showing bonds and geometry, making them valuable for schools and workers handling these compounds.
Safer product development depends on being able to look at a new chemical, sketch it out, and understand where risks could arise—like how a halogen atom might persist in groundwater. Community science efforts and open-access resources help people visualize and grasp the arrangement of atoms, leading to better conversations about health and the environment.
Deep dives into chemical structure matter not only for the curious student, but for everyone making choices about food, health, and sustainability. That sketch of atoms might look dry, but it’s the roadmap for how molecules behave in real life.
Why People Overlook Safety—And Why It Matters
Nobody wakes up wanting to get hurt at work or at home, but people take risks every day while handling products that look innocent on the outside. It's easy to ignore warning labels or training sessions because the risks feel distant, or maybe the product "never caused trouble before." I remember my uncle’s habit of refilling his old pesticide sprayer by hand, thinking gloves were only for the commercials. He got away with it, until he didn’t—ended up with a nasty rash that lingered for weeks. Most accidents happen because folks reach for convenience or speed, forgetting some materials don’t give second chances.
Understanding Common Dangers: Skin, Air, and Eyes
Skin contact tops the list for many chemicals and products. We see people swipe up spills or brush off dust, believing soap and water take care of everything. Depending on the ingredients, direct contact causes irritation, burns, or allergic reactions that don't always show up right away. Eyes take a heavy hit from splashes or particles. One blink and that burning sensation changes your whole day. Inhaling fumes or fine powders brings headaches, nausea, and bigger health problems if it goes on long enough. Manufacturers warn us for a reason, not just to protect against lawsuits.
Steps That Make the Difference
Gloves and goggles sound basic, but a pair that fits right does more work than most people think. Cheap gloves tear, but the right ones act like a real barrier. Goggles that hug the face stop unexpected splashes and drifting dust. For jobs with fumes, a mask or respirator matched to the task belongs on your face, not dangling from your pocket. Ventilation keeps air moving and cuts down on invisible dangers before you even smell them.
Little things keep cleaning products, solvents, or powders from turning into accidents. Every bottle or container has instructions, and following those steps means the product does its job without making trouble. Keep containers closed tight after use—open tops let fumes escape and spill risks climb. Store products on shelves away from food or where kids reach. If something pours or splashes out unexpectedly, cleaning up with bare hands or paper towels raises exposure. Designed wipes or spill kits cost a few bucks but pay off by containing and removing the mess without spreading it around.
Knowledge: The Real Safety Tool
Trusting labels matters. Product names change, formulas shift, and hazards stick around long after the bottle looks empty. Employees and homeowners who take time to learn the real risks behind what they’re using, and who spot warning words like “corrosive” or “inflammable,” protect themselves and everyone nearby. Regular short talks about product handling, even for experienced hands, stop safety culture from turning into pointless paperwork.
Clear zones for using, storing, and disposing of every product make routines safe by default. Washing up thoroughly after each project, even if no obvious dust or residue hangs around, keeps toxic leftovers from following you home. Let experience and a stubborn respect for safety steps lead every step with potentially dangerous products.
Hard Lessons from Chemical Storage
Anyone who has ever spent time around research labs or chemical stockrooms knows chemical storage is all about doing a hundred small things right. Skipping steps usually guarantees ruined samples or worse, safety incidents. After years of handling different ionic liquids, folks learn fast that shortcuts with these materials turn into headaches. 1-Butyl-3-(4-Vinylbenzyl)Imidazolium Chloride fits solidly into this category. Even though its structure sounds technical, its quirks around air, light, and moisture are pretty basic chemistry.
The Enemy: Moisture, Light, and Heat
This compound gets fussy around moisture. Ionic liquids have a strong hunger for water. Even in the driest labs, you can watch bottles sweat after the cap comes off. Water in the bottle spells trouble for long-term stability and reliable results. I’ve seen colleagues try to dry out contaminated batches, wasting time and supplies and ending up with inconsistent reactions. The simplest move uses tightly sealed containers. Screw-cap glass bottles lined with PTFE stoppers keep water out and resist slow chemical attacks from both the contents and the clumsy hands juggling stockroom shelves.
Sunlight also picks favorites in the chemical world, and compounds with vinyl or aromatic groups often fall on the wrong side. Over time, UV breaks bonds and scrambles structures, sometimes yielding new, unpleasant surprises that jam up reactions or turn toxic. Opaque or amber glass bottles knock down that threat. On shelves, the less direct light, the better—locked cabinets or drawers beat out benchtop open air every time.
Heat ramps up problems fast. Some folks dream their samples survive warm rooms or car trunks in midsummer, but ionic liquids like this one tend to creep toward decomposition. Room temperature works for short-term handling. In my experience, a storage fridge around 4°C slows down the chemical clock if the project pauses for weeks. Freezers can help with even longer storage, though it pays to double-bag materials inside sealed containers to block condensation on rewarming.
Labeling and Segregation
Labels sound boring, but missing ones turn any chemical shelf into a guessing game. I keep every bottle marked with the full name, date received, and owner. Detailing hazards right on the container reminds everyone that while these ionic liquids lack the volatility of some solvents, skin and eye exposure should still be avoided. If I see a faded, cryptic bottle on a shelf, I toss it. Guesswork doesn’t belong in chemical storage.
Storing this compound away from strong oxidizers, acids, and bases keeps things calm. I never let ionic liquids share shelves with curious acids or bleach bottles, since spills balloon out of control whenever incompatible groups cross paths. Having a spill kit nearby, including an inert absorbent, adds a layer of quick response if something slips or shatters.
Inventory Habits Make a Difference
It’s tempting to open a fresh bottle and forget about inventory checks for months. I learned the hard way that small leaks or flakes in the stopper let in moisture or cause solidification around the neck. Checking stocks once a month ensures nothing is hiding a problem or running low. Checking for unexpected lumps, cloudiness, or color changes catches issues before they cost real money or research time.
Building Safer Routines
I’ve worked in both academic and industrial labs. The ones with the tidiest, safest storage areas usually didn’t get there by accident. They had good habits and enforced rules. Storing 1-Butyl-3-(4-Vinylbenzyl)Imidazolium Chloride away from light, air, heat, and incompatibles sounds simple, but it only works if everybody on the team buys in. Safe storage preserves chemicals, protects people, and keeps research moving faster. The payoff shows itself in every trouble-free experiment and every “nothing to report” safety meeting.
Looking Beyond the Label
A lot of people see shelves lined with white bottles and think, “Chemical is a chemical.” That isn’t true, not by a long shot. For anyone working in a lab, running a business, or even doing projects at home, the purity and size of a product make a huge difference. I’ve spent my fair share of time in labs and workshops. I know that using the wrong grade can throw off results, mess with safety, or run up costs faster than you’d expect.
Purity Grades: High Stakes for Real Results
There’s a clear reason you can’t bake with just any flour and expect the same bread. Lab work feels the same way. Reagent, technical, food, or pharmaceutical grades each have their own uses and risks. In scientific projects, even a tiny impurity can ruin hours of work. Research shows that students in university labs who use lower-grade chemicals get unexpected results—sometimes failing to repeat published data. That’s frustrating but also costly for both time and equipment. In food or drug manufacturing, contamination leads to expensive recalls and breaks trust. The pharma world sets the toughest standards for good reason. Getting something out the door that isn't what it's promised to be can cause real harm.
The purity question is about safety, too. Impurities can make things more volatile or hazardous—think of cleaning solutions. Low-grade products may work for janitorial tasks but go off-limits anywhere food or medicine is handled. Handling something with an unknown impurity means rolling the dice, and nobody likes betting with their health.
Choosing Package Size: Waste Less, Save More
Running out of material in the middle of a job stinks, but buying too much isn't great either. I learned early on that five-gallon containers are great—until you have to store half of it for months. Chemicals break down. Small shops or school labs don’t have endless storage or fancy ventilation. Big, half-used drums often end up in waste bins, and that's money lost.
Some industries need drum-sized amounts; others just a drop. Giving buyers choices helps reduce waste and lowers the cost of purchase and disposal. I’ve ordered lab supplies in all types of packaging—sometimes a blister pack, sometimes a bulk box. Choosing the right size keeps projects neat and budgets in check. One recent study pointed out that hospitals able to buy medications in smaller vials trimmed both costs and expired waste by as much as 20%—a big chunk when pennies count.
Practical Solutions: Ask, Compare, Double-check
If you ever buy for a lab, shop, or kitchen, keep one thing in mind: never assume all products are the same. Ask suppliers specific questions. “What purity am I getting? Is this size right for what I need this month?” Reliable companies love getting into details and will provide written documentation and safety data sheets for every grade and size they sell. Some, especially established ones, carry years of testing records. Their willingness to back up claims makes a difference.
Don't ignore the people around you either—colleagues, mentors, or online communities can flag trusted suppliers or warn about cut corners. Building that knowledge base brings peace of mind and real savings. There's a certain comfort in knowing what you’re working with is exactly what you paid for.