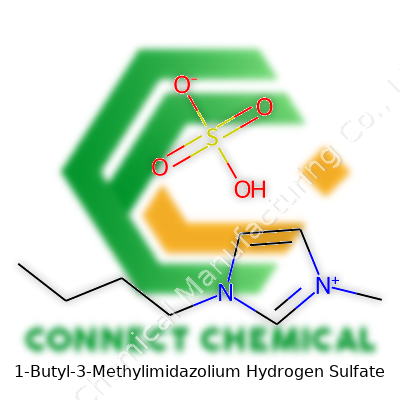
1-Butyl-3-Methylimidazolium Hydrogen Sulfate: A Deep Dive
Historical Development
The story of ionic liquids stretches back over a century, but practical advances took off only in the later decades of the 20th century. 1-Butyl-3-methylimidazolium hydrogen sulfate – or [BMIM][HSO4] in shorthand – belongs to this family that once sat in the realm of laboratory curiosity. Chemists searching for non-volatile, heat-stable solvents in the 1970s and 1980s encountered imidazolium-based ionic liquids. These materials quickly impressed researchers by standing up to aggressive conditions where water and most organic solvents would evapotranspire or decompose. By the 1990s, hundreds of new imidazolium salts entered literature. [BMIM][HSO4] joined the list, opening more options through its relatively easy synthesis and unique compatibility with both organic and inorganic molecules.
Product Overview
1-Butyl-3-methylimidazolium hydrogen sulfate does more than just look good in a chemical catalog. It’s a pale, viscous liquid at room temperature, with a structure featuring a bulky organic cation and an acidic anion. Electrochemists, catalysis researchers, and green chemists turn to this compound for its non-flammability and resistance to evaporation. Unlike old-school organic solvents, it does not contribute to air pollution or workplace volatility. This substance dissolves many types of salts, organic compounds, and even cellulose, which signals real flexibility. In my hands, it never fails to impress during solvent extraction experiments or as a conducting medium in electrochemical trials.
Physical & Chemical Properties
1-Butyl-3-methylimidazolium hydrogen sulfate brings a density of about 1.2 g/cm3 and sits as a liquid down to roughly -50°C. Its melting point hovers below room temperature, so you rarely catch it freezing under normal lab conditions. Viscosity can feel high, demanding patience during pipetting or stirring – a trait that can frustrate, but also prevents messy spills. Odor stays faint and non-irritating, a real step up from many legacy solvents. Water easily mixes in, shifting the ion balance and slightly lowering the viscosity. With moderate thermal stability, it hangs on to structure up to 250°C before breaking down. Ionic conductivities show up consistently strong, which makes this liquid attractive for battery and electrochemical setups.
Technical Specifications & Labeling
Typical chemical supply labeling covers the essentials: chemical name, molecular formula (C8H16N2SO4), purity levels – usually over 99% for analytical work – plus lot number and storage suggestions. Many suppliers assign the CAS number 262297-13-2 to this compound. Batch-to-batch consistency matters, especially since impurities can sharply affect physical properties or reactivity in sensitive syntheses. Many operations choose glass storage because this ionic liquid reacts with certain metals over time, especially at elevated temperatures.
Preparation Method
The go-to route for [BMIM][HSO4] starts from 1-methylimidazole and 1-chlorobutane. These react together, producing the parent bromide or chloride salt first. Following this, a metathesis reaction with hydrogen sulfate anion – often added as concentrated sulfuric acid – swaps out the halide for the desired acid salt. Purification follows: repeated washings, careful removal of unreacted organics, and gentle evaporation under reduced pressure. Water control is crucial, since too much water shifts the balance away from pure ionic liquid and can lower storage stability. Technicians who bypass shortcuts here find themselves with lower yields or impure batches, which hampers downstream success.
Chemical Reactions & Modifications
In the lab, [BMIM][HSO4] has a reputation for behaving predictably, but it reacts when pushed the right way. Mixing with strong bases neutralizes the acidic hydrogen sulfate group, forming other imidazolium salts. Mild oxidants can nudge it towards sulfonate forms. On the organic side, this liquid can support Friedel–Crafts alkylation, acylation, or even support as a solvent/catalyst in esterifications and transesterifications. The parent imidazolium core allows further chain extensions or modifications, leading to a whole family of related ionic liquids with tweaked properties.
Synonyms & Product Names
You’ll often see [BMIM][HSO4] dressed up as 1-butyl-3-methylimidazolium hydrogensulfate, BMIMHSO4, or ionic liquid 104. Suppliers sometimes use proprietary names or abbreviated tags like BMIM HSO4, but the imidazolium base always shows up front in serious specifications. Some journals toss around generalized names like “acidic ionic liquid,” so cross-checking the structure saves you from mix-ups, especially with dozens of similar salts on the market.
Safety & Operational Standards
Lab safety around [BMIM][HSO4] draws most of its rules from the acid content. Skin and eye contact brings irritation, and gloves remain a must. This compound can corrode certain metals, so care in container selection and spill management makes sense. It doesn’t evaporate into the air, reducing inhalation risks, but disposal by dilution or flushing remains a bad idea. Waste management follows local hazardous waste codes, since acidic ionic liquids can disrupt sewage treatment or groundwater health. My experience: strict PPE together with fume hood handling keeps risks low in busy student labs, and clear labeling prevents any accidental mixing with base-sensitive chemicals.
Application Area
What sets [BMIM][HSO4] apart is its chameleon-like behavior in research and industry. Catalysis stands front and center – acid-catalyzed reactions run with better selectivity and reusability than mineral acids allow, and the ionic liquid can sometimes be separated and reused. Extraction chemists tap into its solubility for separating metal ions or rare earths, especially from electronic waste streams. Enzymatic reactions improve in speed and yield for some transformations, as the ionic liquid can stabilize enzymes that would otherwise unfold or stop working in water or alcohol. Battery researchers explore this salt for its electrochemical stability and broad electrochemical windows. On the academic side, [BMIM][HSO4] shines in “green chemistry” projects focused on replacing volatile organics, cutting down emissions, or reducing process waste.
Research & Development
A steady stream of papers examines new applications and properties of [BMIM][HSO4] each year. Developers test new catalysts using this ionic liquid as either a medium or a co-catalyst. Research teams probe its behavior with metals, organics, or biological macromolecules in hopes of developing lower-cost recycling or manufacturing routes. Known for its role in cellulose processing, this liquid dissolves stubborn natural fibers, making it easier to convert wood or agricultural waste to biofuels or commodity chemicals. Pharmaceutical chemists also eye its ability to solubilize poorly water-soluble drugs for improved delivery.
Toxicity Research
Toxicological studies on imidazolium ionic liquids ring a few warning bells but paint a nuanced picture. Acute exposure at low levels tends not to cause severe symptoms, but repeated contact can irritate skin or mucous membranes. Tests in aquatic systems reveal moderate to high toxicity for certain fish and invertebrates, driven in part by the hydrophobic butyl group. Long-term breakdown and ecological impacts remain under review, as persistence in water or soil varies by climate and microbial community. Labs have started shifting to greener disposal protocols and exploring ways to recover or recycle spent ionic liquid to avoid leaks into water systems. As someone who’s had to manage solvents at scale, I always push for containment and thoughtful end-of-life planning, especially for student-run projects or pilot lines.
Future Prospects
Looking ahead, 1-butyl-3-methylimidazolium hydrogen sulfate holds promise for process intensification and greener manufacturing. As more attention lands on non-fossil chemical routes and circular economy models, this ionic liquid finds new homes in plastics recycling, biomass conversion, and pharmaceuticals. Research data on toxicity and biodegradability will shape whether expanded use lives up to current hype. If suppliers can cut production costs and minimize environmental footprints, [BMIM][HSO4] may well lead the next batch of “designer” solvents that help bring academic innovation out of the lab and into the warehouse. With the shift to renewable materials and stricter emissions regulation, practical, versatile, and recoverable ionic liquids like this one could play a leading role. Staying close to the ongoing research pays off for anyone with a stake in clean chemical technology.
What Is It?
1-Butyl-3-Methylimidazolium Hydrogen Sulfate sounds like a chemistry tongue twister, but the story behind this name points to a growing shift in how industry tries to tackle pollution and efficiency. This compound belongs to a family of ionic liquids—a group of salts that stay liquid at room temperature. Unlike regular solvents, these liquids don't evaporate into the air and mess with our lungs. That alone changes how workers deal with chemicals day in and day out.
Why Use It?
Factories use this stuff mainly as a solvent and catalyst when they need to break down tough natural materials or pull metals from ore. In the lab, I’ve watched ionic liquids break down cellulose from wood chips way faster and more cleanly than old-school acid baths. Paper and biofuel companies chase these liquids because they can pull sugar out of plants faster, using less energy and creating fewer byproducts that need cleanup. The U.S. Department of Energy and researchers have numbers to back these claims—ionic liquid-based processes often recover up to 90% of sugar from corn stover, where tradition methods lose a chunk to waste.
Protecting Health and Environment
It’s easy to overlook the health angle. Most solvents in the past came loaded with health warnings—benzene and toluene top the charts for risks. 1-Butyl-3-Methylimidazolium Hydrogen Sulfate won’t join the list of household products, but for those responsible for safety in manufacturing plants, it means less airborne fumes, fewer headaches, and a safer workplace. The environmental angle matters just as much. Regular solvents float into rivers or disappear into the atmosphere, causing damage that lingers for decades. Ionic liquids, including this one, stay put. They don’t evaporate, and with tighter regulations in Europe and the U.S., companies cut back on permit costs and avoid fines.
Challenges and Beyond
Cost always shadows the green chemistry movement. A plant manager will tell you that a gallon of ionic liquid doesn’t come cheap. Companies want to recycle every drop. The tech for reusing ionic liquids still demands more polishing. A study from ACS Sustainable Chemistry & Engineering points at recycling rates above 80% for some ionic liquids after repeated use, but the numbers dip in rougher, scaled-up processes. The best shot for wider use ties directly to supporting research into cheaper production routes or developing ways to remove stubborn impurities after processing.
Some operations also worry about what happens if these compounds do hit water, since very little data covers their long-term effects in nature. Down-to-earth, the solution sits with better containment and new cleanup tech. Environmental chemists need time and money to chase what happens in streams and soils after release. Until then, the full story isn’t written, and caution beats headlines touting miracle solutions.
The Road Ahead
Every industry tries to walk the line between efficiency, cost, and care for the world outside the gate. 1-Butyl-3-Methylimidazolium Hydrogen Sulfate reveals how far things have come since toxic solvents ruled the landscape. With smart tweaks, wider education, and more focus on green chemistry, more plants in textiles, biofuels, and metal recovery fields could make the jump. Real change often grows from these hidden advances—less hype, more results, and a safer place for folks who make the products we use.
Understanding What’s On Your Workbench
It’s easy to get swept up by labels like “green solvent” or “ionic liquid” and assume everything in a lab setting comes with fewer risks. 1-Butyl-3-methylimidazolium hydrogen sulfate pops up in journals as a promising game-changer in synthetic chemistry, clean energy, and even waste recycling. It doesn’t evaporate like traditional solvents. It pushes reactions along at room temperature, sometimes skipping the need for harsh catalysts. Still, day-to-day safety means more than performance stats on paper.
I’ve worked with imidazolium-based liquids through several projects. Their greasy texture and low odor can feel benign ― especially compared to classic acids like sulfuric or solvents that burn your nose before the bottle is unscrewed. That almost always proves to be a mirage if you drop the usual sense of caution. This compound brings together an acidic ion and an organic base: you get the same corrosive punch you’d expect from hydrogen sulfate and the stubborn ability of organics to soak through skin.
Where Risks Show Up
Direct contact remains a hazard. Chemical safety databases show that 1-butyl-3-methylimidazolium hydrogen sulfate irritates eyes and skin. If exposure goes unchecked, someone could wind up with chemical burns or persistent dermatitis. Lung exposure isn’t benign either — these ionic liquids can aerosolize under strong stirring or as fine droplets, and breathing in even tiny amounts can irritate your airways. The risk ratchets up if you have any open cuts or cuts from broken glassware.
Some early studies on imidazolium-based salts show they can disrupt biological membranes if given the chance. In 2013, research published in the journal Ecotoxicology reported negative effects on simple aquatic organisms. While long-term toxicity in humans doesn’t draw as much media attention, we’d be wise not to treat these liquids like household cleaner.
It’s Not a Question of “Toxic” or “Non-toxic”
Some colleagues treat any “green chemistry” tool as harmless, but safety isn’t a binary switch. Gloves, goggles, and lab coats belong every time the bottle comes out, even for small-scale work. Nitrile gloves stop these liquids just fine; bare skin doesn’t. Fume hoods help limit the chance of breathing in mists. Cleaning spills means mopping up with plenty of water and neutralizing any remaining acid, but I never rely on paper towels alone—plastic or rubber spatulas give far more control over sticky, oily spills that refuse to wipe away. Closed containers cut down on accidental exposure.
Disposal brings its own headaches. Municipal drains and trash bins can’t handle ionic liquids, as wastewater plants don’t break them down. Most labs send spent solutions out for hazardous waste processing. Anyone who’s ever tried cleaning up after a leaky bottle of this stuff learns the hard way what “persistent” really means.
Possible Steps Toward Safer Handling
Regular training drills keep habits sharp. I see labs get complacent and forget that new students or employees join in every semester. Written safety sheets matter, but nothing sticks like walking through what to do if skin gets splashed or a beaker tips over. I keep extra gloves by the door and pour out only a working amount, treating the rest as untouchable stock—after all, refilling a little costs less than cleaning a big mess.
New substitutes enter the market every year, but for now, 1-butyl-3-methylimidazolium hydrogen sulfate earns treatment as a “handle with respect” substance. It pushes chemistry forward—and it sticks to fingers, gloves, and glass just the same. That familiarity tempers any shortcut or assumption of safety.
Getting to Know the Molecular Structure
Many of us working in labs or industrial chemistry have crossed paths with ionic liquids at some point. Among them, 1-Butyl-3-methylimidazolium hydrogen sulfate has slipped into everyday chemical processes. If you’re staring at the name and hoping for a quick answer, the chemical formula you are looking for is C8H16N2O4S.
Why Structure Matters in Everyday Labs
Ionic liquids like this one carry a special status. They offer unique properties, bringing both organic and inorganic worlds together. The structure includes the 1-butyl-3-methylimidazolium cation and the hydrogen sulfate anion. The imidazolium ring isn't just for show — it delivers good thermal stability, which lets the compound stand up to demanding tasks. If you’ve ever needed a solvent that won’t evaporate or catch fire easily, you can see the practical side right away.
From Green Solvents to Catalysts
I first encountered this compound during a project focused on green chemistry. We needed a solvent that would dissolve a range of organic molecules without stinking up the lab or requiring special ventilation. 1-Butyl-3-methylimidazolium hydrogen sulfate stepped up as a non-volatile choice. It didn’t pose the same dangers as the old-school volatile organic compounds, and it cut down on hazardous waste.
This chemical’s unique structure lets it serve as both solvent and acid catalyst in reactions like esterifications or alkylations. The hydrogen sulfate part provides a natural acidity, pushing reactions forward without needing a second additive. Reactions ran smoother, and product yield often improved. I know some chemists prefer the simplicity of traditional acids, but tools like this make tougher work easier, especially when handling sensitive or tricky substrates.
Tough Questions About Safety and Environment
Not every new chemical ends up making life easier. Ionic liquids like this one stirred hope for less toxic industrial practices. Still, questions remain. They don’t evaporate and won’t fill the air with fumes, which many saw as a blessing. Yet, disposal isn’t simple. These compounds can bioaccumulate, so keeping them out of waterways makes sense. Fact: recent studies from environmental agencies tracked a few ionic liquids in soil samples and suggested some biodegradability, but not enough yet to give a free pass.
I always urge colleagues to look past the hype and ask direct questions: Can your team collect and recycle the liquid? Does your supplier provide solid data on purity and composition? There’s still a lot to learn. Real improvements come from honest testing, not just reading supplier brochures.
Solutions for Responsible Use
Easy solutions rarely land on your desk in chemical safety. One option: set clear collection protocols for ionic liquid waste and push for closed-loop recycling methods. Teaming up with academic labs for toxicity screening helps, too. If you find yourself on the fence, start small and build experience. Track your waste, keep records, and reach out to researchers who’ve handled these compounds for years.
Trust in any chemical comes from clear results and honest evaluation, not just trends. Whether using 1-butyl-3-methylimidazolium hydrogen sulfate as a catalyst or tech solvent, knowing the formula and respecting its strengths — and risks — paves the way for better chemistry.
Understanding the Substance
1-Butyl-3-methylimidazolium hydrogen sulfate—often seen in labs and many chemical processes—has a stable ionic liquid nature but carries a serious bite if handled carelessly. I remember the first time I encountered it on a university research team. Our supervisor warned us that careless storage can quickly lead to more problems than you bargain for. Since then, I’ve kept an extra eye on how and where this liquid ends up.
Why Safe Storage Matters
People sometimes underestimate chemicals that don’t have a nasty smell or an obvious hazard label. This compound, though, brings both corrosive and hygroscopic traits to the table. It absorbs moisture from the air, which can mess with both its purity and safety. Moisture can even break it down, and its acidity can eat away at unsuitable containers or bench tops. Accidents or leaks ruin not only the chemical but also your day and possibly your project budget.
Solid Ground Rules for Storage
The first thing everyone should focus on is the container. Glass with a proper sealing cap always sits at the top of my list. Plastics might break down; metals won’t handle the acidity. Room temperature works most of the time—just don’t let things get too warm or too cold. Sudden shifts in temperature can lead to condensation. Moisture and this liquid do not mix.
Controlling humidity plays a bigger role than most people think. Dry, dark cupboards do more to protect this stuff than any high-tech fridge can. A simple desiccator, used by most chemists, keeps water away reliably enough. I’ve used those little silica gel packs in a pinch when gear ran short, and it saved expensive material more than once.
Labeling and Segregation
Every storage cabinet needs a clear labeling system. It’s a habit that pays off every single time someone new joins your team or when someone’s memory fails. Corrosive chemicals like this one should always sit away from basic or reactive substances. I’ve seen accidents just from bottles sharing space that never should have. Mixing can trigger releases and unexpected reactions, even under “perfect” conditions.
Personal Experience and Industry Best Practices
I’ve watched busy labs cut corners, storing ionic liquids next to bleach or solvents. Sometimes things go fine, until that one day someone forgets to close a lid and humidity takes over. At my previous job, regulations evolved after a costly spill, forcing us to use lockable, ventilated storage for every container. OSHA and the CDC urge similar steps because corrosives have sent people to the hospital over small missteps.
Chemical storage doesn’t always get the headlines or funding, yet a little investment—both in time and materials—prevents costly mistakes. Lab managers who keep simple checklists around reduce risk every single week. Clear protocols, routine checks, and a little old-fashioned paranoia help chemicals like 1-butyl-3-methylimidazolium hydrogen sulfate stay as harmless as they look in the bottle.
Steps Worth Taking
Separate your chemicals, label containers clearly, keep humidity at bay, use the right containers, and make protocols a real habit, not just something for audits. All of it adds up, making daily work safer and projects smoother. Chemical safety shows up in small acts done every day, not just rules on the wall.
Seeing Beyond the Buzz: A Closer Look at This Ionic Liquid
Digging into the chemistry world, I keep running across names like 1-Butyl-3-Methylimidazolium Hydrogen Sulfate, better known in labs as [BMIM][HSO4]. You often see it in the context of “green” chemistry, promising answers to the problems from older toxic solvents. Anyone who’s spent time cleaning up after a spill of chloroform can appreciate why folks like the idea of safer alternatives.
Living With Water… and Alcohols
[BMIM][HSO4] stands out for its strong relationship with water. It dissolves readily, skipping any drama over solubility, and can handle a good mix with short-chain alcohols like methanol and ethanol. Some might take this for granted, but for process work, it’s a real bonus. Running extractions with water or simple alcohols becomes way less picky. It’s a bit like working in a kitchen where the utensils always fit the job—everything just works together.
Things That Don’t Mix
Many organic lab staples, such as hexane, toluene, or diethyl ether, don’t have the same cozy relationship with [BMIM][HSO4]. Try mixing in a non-polar solvent, and you get stubborn separation. This mismatch steers a lot of separation science. It can make [BMIM][HSO4] useful for certain phase-transfer setups but also warns you off if you’re hoping for a single-phase zone with these solvents. Before you risk precious material or waste hours troubleshooting, it's worth remembering: ionic liquids formed with bulky anions like hydrogen sulfate rarely just slip into apolar company.
Industrial Reality Checks
In industries like pharmaceuticals or fine chemicals, solvent compatibility means more than clean mixing. It can spell higher yields, safer work, and fewer headaches cleaning up. [BMIM][HSO4] doesn’t evaporate much, making it handy for reactions run at a little heat, and it won’t start fires like old-school ethers do. On the downside, if you switch to hydrocarbons mid-process, you’ll hit a wall, creating unwanted layers and complicating recovery steps.
Environmental Considerations
With stricter rules on emissions and workplace safety, a solvent like [BMIM][HSO4] earns its spot by staying put and not making the air hazardous to breathe. Mixing it with water or alcohol helps with treatment and recycling. If a facility can keep most work in that solvent zone, life gets simpler. Workers and local regulators both avoid surprises.
Don’t Take Shortcuts
Every chemist learns the hard way: chasing after “universal” solvents leads to dead ends. [BMIM][HSO4] works beautifully in systems rich in polar, protic solvents—think water, methanol, ethanol. Pair it with DMSO or acetonitrile, and you’ll usually get a single fluid mix, opening doors for catalysis or biotransformations. Some teams use this to cut steps from multi-phase processes and save on energy or waste disposal. That takes coordination: chemists, engineers, and EHS folks working with a clear understanding of what mixes and what won’t.
Better Outcomes Through Testing and Documentation
Running solubility tests remains non-negotiable. In my work, nothing beats a small-batch mix-and-see trial. Most issues happen when people lean too hard on tables or reports from the literature and skip that hands-on check. Documenting solvent compatibility for [BMIM][HSO4] means not just listing the winners and losers but also watching for surprises under different temperatures, concentrations, or impurities. The payoff is a smoother scale-up—and fewer late-night phone calls when a process starts misbehaving.