1-Butyl-3-Methylpyridinium Tetrafluoroborate: An In-Depth Perspective
Historical Development
Research into ionic liquids took a leap forward in the late twentieth century. Early ionic liquids relied heavily on imidazolium and pyridinium derivatives. Chemists in the 1990s started exploring structure-property relationships, looking for salts with high thermal stability, low volatility, and the ability to dissolve diverse materials. One breakthrough was the development of 1-butyl-3-methylpyridinium tetrafluoroborate, a compound now appreciated in labs and industry alike. This compound’s synthesis stemmed from the need for more robust ionic liquids that could resist hydrolysis and chemical breakdown. From the moment it arrived on the research scene, it stood out for its blend of durability and handling ease.
Product Overview
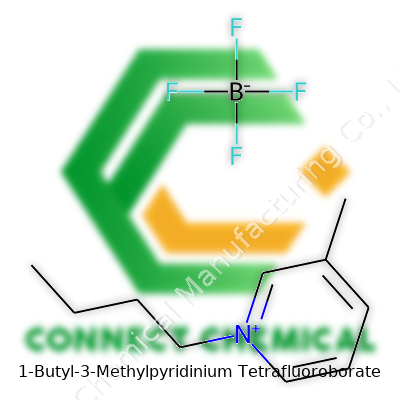
1-Butyl-3-methylpyridinium tetrafluoroborate belongs to the family of pyridinium-based ionic liquids. This salt consists of a pyridinium ring, functionalized with a butyl and a methyl group, paired with a tetrafluoroborate anion. Its liquid state at room temperature and manageable viscosity make it suitable for advanced applications, from catalysis to electrochemical devices. Researchers and engineers appreciate its ability to serve as a solvent or electrolyte, often outperforming more traditional organic solvents due to its stability and low vapor pressure.
Physical and Chemical Properties
The liquid’s pale yellow or colorless appearance makes it easy to handle. Its density often falls around 1.08–1.18 g/cm³ at room temperature, and it resists decomposition under standard conditions. With a broad electrochemical window, the compound remains electrochemically inert over a wide range of potentials. This makes it a top pick for batteries and capacitors. Its moderate viscosity supports ion mobility without being unwieldy in pumps or mixing. The salt dissolves various organic and inorganic compounds without the toxicity or volatility seen in old-school solvents. Chemical resilience against water and oxygen extends its shelf life and broadens its usability.
Technical Specifications & Labeling
Labs typically receive this chemical in amber bottles, labeled to emphasize its purity (often 99% or higher) and moisture sensitivity. CAS number, molecular weight, and batch number appear on every shipment to track quality. Detailed safety information and recommended storage conditions travel with the material, and suppliers provide certificates of analysis to verify precise identity and purity.
Preparation Method
Synthesis of 1-butyl-3-methylpyridinium tetrafluoroborate generally starts with 3-methylpyridine and 1-chlorobutane. Through an alkylation process, butyl and methyl groups attach to the pyridinium ring, followed by metathesis with tetrafluoroboric acid or sodium tetrafluoroborate. The process often involves careful purification through washing and recrystallization, with water and organic solvents used to separate impurities. Labs that invest in glove boxes or moisture-controlled hoods avoid hydrolysis during preparation, which speaks to the importance of technical know-how in synthesizing high-purity products.
Chemical Reactions & Modifications
This ionic liquid can undergo modifications to tweak solubility or catalytic properties. For example, altering the alkyl group length changes viscosity and hydrophobicity, giving researchers tools to fine-tune mixtures for specific industrial or laboratory tasks. Chemical reactivity with strong nucleophiles remains low, allowing the liquid to serve as an inert medium for sensitive transformations. It helps promote palladium-catalyzed coupling reactions and sometimes even outperforms organic solvents thanks to efficient phase transfer and stabilization effects. Its chemical structure also allows easy recycling in multi-step processes, which reduces waste.
Synonyms & Product Names
Some suppliers label this chemical as N-butyl-N-methylpyridinium tetrafluoroborate or BMPy BF4. You’ll often run into abbreviations like [BMPy][BF4], which show up in scientific literature and on packaging. The variety of names reflects different naming traditions around the world, but the compound stays the same. A little vigilance prevents confusion between similar-sounding pyridinium products, a lesson any experienced chemist learns quickly.
Safety & Operational Standards
Labs and plants that use ionic liquids like 1-butyl-3-methylpyridinium tetrafluoroborate prioritize safety. This compound stands out by lowering fire and explosion risks, lacking the high flammability of common organic solvents. Workers still wear gloves, goggles, and lab coats, since skin or eye contact can cause irritation. Material safety data sheets warn against inhaling vapors or ingesting even small quantities. Local ventilation and careful waste handling keep workplace exposures low. Spills respond best to absorbent materials, with thorough cleaning to avoid residues. Since ionic liquids can persist in the environment, users discuss proper disposal with waste handlers, building on the chemical sector’s push for safer, greener practices.
Application Area
People exploring new battery chemistries often rely on this compound as an electrolyte because of its resilience to breakdown and its support for wide voltage ranges. Its role extends into sensor development, organic synthesis, and even as a medium for carbon capture experiments. Electroplating sees steady usage, with the ionic liquid supporting uniform deposition of metals at lower temperatures than legacy solutions. Pharmaceutical researchers study it as a solvent for biologically active compounds when traditional organic solvents fail due to toxicity or solubility issues. Its resistance to evaporation gives it a big edge in high-temperature processes, and its inertness benefits bio-refining and materials recycling.
Research & Development
Academic groups and chemical companies both push to expand the use of ionic liquids. Recent years saw a surge in published papers focused on improving synthesis routes, finding more cost-effective anion sources, and lowering environmental impact. Some scientists publish on ways to recover and recycle the liquid after use, which addresses both sustainability and operating costs. Cutting-edge research into supramolecular chemistry relies on this ionic liquid for controlled self-assembly studies. It finds a role in scaling up electrochemical devices, bridging the gap between lab-scale testing and commercial products. The ongoing search for renewable energy storage and green chemistry relies on this salt as a dependable testbed material.
Toxicity Research
Debate around the toxicity of ionic liquids remains lively. Early claims of benign environmental impact gave way to closer scrutiny. Studies suggest that while 1-butyl-3-methylpyridinium tetrafluoroborate poses fewer inhalation risks than volatile organics, it may exert toxic effects in aquatic environments if spilled. Researchers measure acute toxicity in fish and invertebrates, guide industry in keeping releases to a minimum, and target ways to break down any waste with chemical or biological treatment. Its low vapor pressure means little ends up in the air, but long-lived residues in waste streams demand responsible handling. Toxicologists and regulatory agencies keep a close watch, adapting standards as more data emerges from lab observations and real-world incidents.
Future Prospects
Research teams keep finding new directions for this compound. As batteries move away from traditional liquid electrolytes, demand grows for ionic liquids that handle high voltage, elevated temperature, and long shelf life. The search for greener, cost-effective production routes continues, with some groups turning to bio-based raw materials. Waste treatment firms look for methods to safely degrade spent ionic liquids, balancing growing usage with environmental responsibility. In recycling and materials science, new techniques for extracting valuable metals rely on the selectivity and durability of this liquid. Exploring combinations with other ionic liquids or nanoparticles could unlock next-generation sensors, membranes, and separation processes. Its role in supporting safe, sustainable chemistry looks set to expand as more people recognize what it can offer.
Getting to Know the Chemical
Most people probably haven’t crossed paths with 1-Butyl-3-Methylpyridinium Tetrafluoroborate in daily life, but if you spend any time in a chemistry lab or the deeply technical side of industry, this ionic liquid might ring a bell. This isn’t something you’ll find under the kitchen sink—it’s familiar to researchers busy trying to solve tough problems in energy, electronics, and even pharmaceuticals. For me, seeing friends sweat over electrolyte stability during their PhD work opened my eyes to just how central these liquids can be.
Green Chemistry Grows Up
Talking with scientists who pay attention to the planet, I hear the same thing: there’s a desperate need for safer, more efficient solvents. 1-Butyl-3-Methylpyridinium Tetrafluoroborate doesn’t evaporate into the air or burn easily, making it more appealing than volatile organic solvents. In fields like organic synthesis, researchers lean into this compound because it lets them push reactions further, collect higher yields, and avoid the headache of dealing with toxic fumes. Studies from the Royal Society of Chemistry back up those claims, showing improved process efficiency and less environmental harm by using ionic liquids like this one.
Electrolyte Workhorse
Anyone who has cracked open a battery knows the electrolyte inside keeps the lights on—literally. Engineers working on advanced lithium-ion batteries and supercapacitors have been pressing for safer, longer-lasting electrolyte solutions. I remember a college project where we tried every cheap option; fire risks and leaks haunted every experiment until a mentor handed over a vial of ionic liquid—pure game changer. 1-Butyl-3-Methylpyridinium Tetrafluoroborate doesn’t catch fire, supports a wide electric window, and keeps batteries humming over lots of recharges. Researchers at MIT keep pointing to these properties as reasons for using this liquid in next-generation energy storage.
Cleaning Up Difficult Tasks
Industrial chemists have found this compound helps pick apart mixtures in tricky separations. Refineries and pharmaceutical makers rely on ionic liquids to strip out impurities or extract key ingredients—tasks that water or regular solvents simply can’t match without ugly side effects. Early on, my own stint at a specialty chemicals firm showed the costs and headaches that follow from solvent spills. Making the switch toward ionic liquids opened new ways to recover spent materials, lowering hazardous waste hauling and compliance pressure.
Barriers, Risks, and Responsible Use
No chemical is a silver bullet. 1-Butyl-3-Methylpyridinium Tetrafluoroborate still needs careful handling. It isn’t as cheap as legacy solvents, and anytime you start scaling up something new, you run into questions about disposal, toxicity, and long-term effects. I’ve seen companies stall out mid-rollout, stuck between hype and practical realities. Papers in Environmental Science & Technology have also called for deeper study into how breakdown products from such compounds spread in soil and water.
What Comes Next?
Holding onto every step forward in green chemistry takes open dialogue and ongoing testing. Researchers, industry leaders, and policymakers all stand to gain from sharing data on long-term impacts—and not hiding nasty surprises. More transparency will mean better protection for both workers and the environment. As companies increase adoption, investments in recycling and material reclamation processes will allow ionic liquids like 1-Butyl-3-Methylpyridinium Tetrafluoroborate to truly deliver their promise without leaving a new pile of problems behind.
Understanding the Formula and Weight
1-Butyl-3-Methylpyridinium Tetrafluoroborate isn’t something you run into every day, unless you’re comfortable around ionic liquids. Its chemical formula looks like this: C10H16BNF4. Dig into the numbers and you’ll see it has a molecular weight of about 237.05 g/mol. That unique mix of carbon, hydrogen, boron, nitrogen, and fluorine gives it more punch than just a jumble of elements.
The Real-World Value
There is a rising demand for cleaner, greener approaches in chemistry labs and manufacturing floors. 1-Butyl-3-Methylpyridinium Tetrafluoroborate steps up as a strong option for those hunting for an alternative to volatile organic solvents. It barely evaporates, doesn’t ignite easily, and can often be reused after simple purification. These points matter when scientists look to cut down on hazardous waste and unpredictable working conditions.
What drew me to ionic liquids like this one was a need for solutions that actually lower the risk of fires in a research lab. Those risks creep up fast, especially in older buildings filled with recycled equipment and sleepy ventilation systems. One spill or open flame, and the work could end in disaster. Switching to an ionic liquid changed that game in our space. That alone justifies the hunt for new molecules.
Challenges in Widespread Adoption
No breakthrough comes without its bumps in the road. 1-Butyl-3-Methylpyridinium Tetrafluoroborate isn’t produced in huge batches, so pricing can intimidate smaller labs or startups. Large chemical manufacturers look for stable sourcing and supply. Besides, even a chemical that’s less hazardous up front still deserves careful disposal. Tetrafluoroborate-based ions, in the wrong conditions, could break down to release tiny amounts of corrosive hydrofluoric acid—nothing you’d want wandering through a city water system.
Reports have shown that ionic liquids sometimes don’t break down easily in the environment. That poses its own puzzles for those of us who want to keep research sustainable. A study I read back in 2022 showed that while the pyridinium cation can sometimes get chewed up by soil bacteria, the tetrafluoroborate anion sticks around. Experts keep an eye on this, pushing for testing before full-scale rollouts.
Finding Smarter Solutions
Instead of giving up, chemists keep poking and prodding for answers. Tweaking the molecular structure or swapping the anion for something less persistent may ease worries about toxicity and environmental impact. Teams can also use closed-loop systems. That means carefully collecting, cleaning, and reusing the liquid—cutting down the risk of polluting local ecosystems.
Government-backed research helps smooth the pathway for these advances. Grants pave the way for safer disposal methods, analytical testing, and lifecycle studies. None of these improvements fall from the sky. They all stem from a mix of pressure—legislation, market demand, and that feeling everyone in the lab gets when they know their work leaves a mark, for better or worse.
Looking Ahead
As the world watches for safer, sustainable chemicals, facts matter. Understanding exactly what goes into 1-Butyl-3-Methylpyridinium Tetrafluoroborate—down to the chemical formula and its weight—gives people on the ground more power to ask the tough questions and make informed choices, both for science and for daily life.
Understanding the Risks
If you work in chemistry labs or are interested in ionic liquids, you have probably come across 1-butyl-3-methylpyridinium tetrafluoroborate. This compound pops up in research around catalysis, batteries, and electrochemistry. Scientists and technicians like the low volatility and high thermal stability, but ignoring safety can bring trouble fast.
The chemical formula might look harmless, but this stuff can irritate skin, eyes, and the respiratory tract. Pyridinium-based ionic liquids sometimes carry toxicity concerns because long-term effects are still under review. My own run-ins with similar compounds taught me something important: just one careless splash or spill, and you’ll wish you took precautions seriously. Gloves and safety goggles aren’t suggestions, they’re requirements.
Why Safe Handling Matters
Lab safety isn’t some academic exercise or bureaucratic hoop. Inhaling dust from liquid spills may cause coughing, shortness of breath, or dizziness. My colleague once described the burning sensation after touching ionic liquid residue, and that story stuck with me. Every lab worker has stories about a forgotten mask or lazily tied apron making a bad day worse. These experiences shape why safety guidelines exist—and why people follow them with respect, not just compliance.
Beyond immediate irritation, ionic liquids with tetrafluoroborate anions can release toxic gases if exposed to moisture and strong acids. That includes boron trifluoride and hydrogen fluoride, both of which demand ventilation and containment. Facts from NIOSH and OSHA underline the risk: hydrogen fluoride exposure ranks as one of the top chemical hazards due to tissue damage and bone toxicity from repeated contact.
Effective Safety Measures
Good habits start with clear labeling and secure storage. Clean lab benches and quick response to spills keep little accidents from snowballing. Every time I handle this ionic liquid, I double up on nitrile gloves, check that my splash goggles are snug, and use a chemical fume hood. Good airflow cuts down on vapor risk and means you won’t end up coughing through a procedure. Safety showers and eyewash stations should be nearby—accessible and functional, not gathering dust or blocked by clutter.
Disposal isn’t just about dumping waste in the right drum. I’ve seen containers swell up or leak because somebody didn’t cool their waste or failed to seal things tightly. You can’t treat ionic liquids like water-soluble trash. Designated containers, labeled and stored in cool, dry conditions, matter just as much as the steps you take during experiments.
Training and the Bigger Picture
Manufacturers include datasheets for a reason, but real-world training brings the rules to life. Regular safety drills, checklists, and peer reviews make safety second nature, not a box to check. Good science happens when everyone trusts their lab partners to look out for safety just as much as results. Institutions and companies do best when they foster this trust, investing in personal protective equipment, up-to-date SDS access, and regular training sessions.
Personal experience, regulatory facts, and company policies align on one point: treat new chemicals with care. 1-butyl-3-methylpyridinium tetrafluoroborate has perks in high-tech research, but these mean nothing without respect for safety. Every careful glove change and careful label translates into workers heading home healthy—day after day, experiment after experiment.
Understanding What You’re Keeping on the Shelf
1-Butyl-3-methylpyridinium tetrafluoroborate isn’t the most ordinary chemical. People use it as an ionic liquid, often for its stability and low volatility. Even though it doesn’t boil away the way some solvents do, a few missteps with storage can quickly turn safe handling into a headache. I’ve watched long-forgotten bottles turn hazardous simply due to poor storage habits, and cleaning up after that is not an experience anyone ever enjoys.
No Sunbathing for This Compound
Direct sunlight just doesn’t mix with most chemicals, and ionic liquids aren't exceptions. The bottles should live somewhere out of the sun. I’ve worked in cluttered labs where skylights seemed like a nice idea—right up until you notice that your liquids are changing color or creating odd smells. Heat speeds up unwanted reactions, sometimes even with chemicals that seem stable day to day. For 1-butyl-3-methylpyridinium tetrafluoroborate, think of any bright, warm shelf as off-limits. Stick to cool, shaded storage.
Humidity Is an Enemy
Ionic liquids often draw moisture from the air. This one can take up water and shift its properties—sometimes it’ll even ruin an experiment that counts on low moisture. Sealed containers help. Nothing fancy, just a bottle with a proper screw-cap works—if in doubt, grab some parafilm and seal the opening. I’ve seen people get lazy and leave lids loose, figuring “it’ll be fine for a day or two." It’s never fine. Once water sneaks in, you can’t always get it back out, and the cost of wasted chemicals climbs fast.
Choosing the Right Containers
Plastic and glass both work, but not all plastics hold up over time. Some can leach, crack, or let vapor escape. I always trust glass with a clear chemical compatibility chart. Label the container with the compound’s full name, the date received, and any hazard warnings—complacency leads to mix-ups, especially if storage shelves get busy. Nothing ruins your week faster than trying to recall which unlabeled bottle holds the expensive ionic liquid and which one needs to go in the waste stream.
Keep It Away from Reactive Stuff
This chemical plays nice with many materials, but that doesn’t mean you toss it next to strong acids or bases. Store it away from anything you know reacts with boron or fluorine. I keep a habit from my lab days—double-checking what sits on the shelves above and below any reactive chemical. Spills, breakages, or leaky caps can start dangerous mixtures before you know what happened. If it’s practical, a separate secondary container or tray catches any drips or leaks, saving cleanup time and lowering risk.
Don’t Let Waste Build Up
Unused samples or residues deserve just as much care. Trying to tuck them into a corner—hoping to “deal with it later”—always backfires. Get a clear protocol for disposing of or treating small leftovers. Some facilities track this closely, printing out disposal logs and weighing bottles before and after sampling. I've done it with a notebook and a good pen, which works just as well for labs without fancy tracking systems. Don’t wait until someone asks, “Hey, whose bottle is this?”
Why Good Storage Is More Than a Rule
People might glance at storage rules and think of them as red tape, but they keep lab work predictable and a lot less expensive. Proper handling saves time, reduces mistakes, and protects health. The best labs don’t just have written guidelines—they’ve got everyone looking out for each other, picking up on the little details that keep every bottle where it belongs and every experiment on track. If you respect what goes in the bottle, you’ll get better results—and a lot more peace of mind every day.
Moving Past Traditional Solvents
Years in the lab teach you that finding the right solvent changes everything. 1-Butyl-3-Methylpyridinium Tetrafluoroborate, often called a pyridinium ionic liquid, feels like a game changer for chemists and engineers who need more than what water or acetone can offer. Its low volatility and ability to dissolve both organic and inorganic substances set it apart from the usual choices. Regular solvents can evaporate, making them risky and harder to contain, but this ionic liquid stays put, even at higher temperatures. That helps keep work spaces safer and product yields steadier.
Advancing Green Chemistry
Working in green chemistry circles, I saw colleagues frustrated with the environmental costs of common solvents—solvents that damage air quality and add to hazardous waste. Pyridinium ionic liquids like this one opened new doors. Their stability and low flammability mean less worry over accidental fires or hazardous fumes. That’s part of why industrial labs looking to lower waste or step toward greener certification put these compounds on their radar.
Researchers from the American Chemical Society have pointed to ionic liquids as being key in making chemical processes more sustainable and efficient. Shifting away from volatile organics cuts down on solvent loss, which matters both for health standards and for controlling costs over time.
Electrochemistry Gets a Boost
Electroplating and battery research demand materials that conduct electricity without breaking down. Traditional aqueous or organic electrolytes bring corrosion and evaporative loss into play. 1-Butyl-3-Methylpyridinium Tetrafluoroborate serves as a robust electrolyte, resisting breakdown even under harsh voltages. Lithium batteries, capacitors, and fuel cells rely on electrolytes that don’t degrade—they need to last for years inside a device. I’ve seen startups and big tech firms alike experiment with these ionic liquids to push battery life and efficiency higher.
Research at Argonne National Lab and similar institutions demonstrates that ionic liquids give higher thermal stability and wider electrochemical windows than conventional choices. That opens the path for new devices—smaller, lighter, and safer than their predecessors.
Catalysis and Separation Processes Take a Step Forward
Some of the best stories come from process chemists talking about how long it used to take to separate product mixtures or run catalyzed reactions. 1-Butyl-3-Methylpyridinium Tetrafluoroborate often plays a double role here: solvent and co-catalyst. Its ionic nature changes the reactivity of other molecules, often speeding up transformations or making purification less of a headache. Bio-based feedstock processing, like turning agricultural waste into fuels or valuable chemicals, gets a lift from this kind of solubility and selectivity.
Studies funded by the EU and industrial partnerships also recognize ionic liquids’ value in dissolving cellulose, a notoriously stubborn plant polymer. That opens the door for more efficient biofuel production, something that matters for nations trying to cut dependence on petroleum. Instead of harsh acids or potent alkalis, these ionic liquids bring a milder approach without sacrificing yield.
Room for Growth and Remaining Challenges
Handling cost remains a hurdle—large-scale production and recovery still outpace some budgets. Recyclability also matters: closed-loop systems using the ionic liquid as both processing aid and product separator show promise but demand investment in equipment and training. If companies back continued research, expect to see these materials working not just in the lab, but throughout large-scale manufacturing, electronics, and green energy projects.