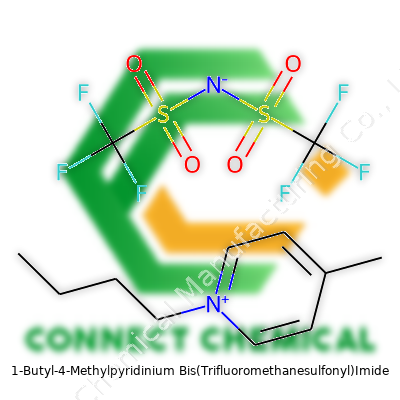
1-Butyl-4-Methylpyridinium Bis(Trifluoromethanesulfonyl)Imide: An In-Depth Commentary
Historical Development
Curiosity for room temperature ionic liquids exploded in chemical circles during the last two decades of the twentieth century. Word spread among research labs that alternatives to traditional volatile solvents could change the way we handle everything from synthesis to separation. Among the stars of this movement, 1-butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide, sometimes called [BMPy][Tf2N], became a name that crop up when people talked about flexible, non-hazardous processing. The journey started with a focus on changing only the cation—tinkering with the pyridinium ring by attaching a butyl and a methyl group—leading to a compound with unique stability. Buffeted by discoveries about the benefits of the bis(trifluoromethanesulfonyl)imide anion, chemists realized this approach could knock down viscosity, increase chemical window, and suppress water absorption. That kind of innovation didn’t just interest researchers; companies jumped at the prospect of greener solvents, which reshaped the thinking in laboratories and industry worldwide.
Product Overview
1-Butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide belongs to a class of ionic liquids that ditch the typical headaches associated with volatile organic compounds. The salt remains liquid at room temperature, showing a viscous, colorless to pale yellow appearance. Because it doesn’t give off noxious fumes, labs can streamline handling and storage. Each batch comes labeled for purity, water content, and specific use-cases, responding to the strict checks required for advanced applications. You’ll see this compound packaged in glass under tight seal, since it reacts fast with moisture in the air. My team once ordered a sample only to discover specks of cloudiness—a lesson in how even trace contaminants change performance in electrochemistry.
Physical & Chemical Properties
This ionic liquid carries a density around 1.4 g/cm³ near room temperature, heavier than your average solvent. Viscosity reaches up toward 65 cP at 25°C—sticky, but manageable for coatings or extractions. The high thermal stability lets it work up to 350°C before serious decomposition sets in, a property that has drawn attention from engineers pushing boundaries in thermal batteries and advanced lubrication. Conductivity can reach 4-6 mS/cm, depending on exact conditions and purity, showing it's no slouch for use in batteries or fuel cells. As for hydrophobicity, the [Tf2N] anion blocks most water from dissolving, so the compound shrugs off humidity far better than imidazolium-based cousins. Try to dissolve it in water, and you’ll find distinct separation. Put it with less polar organics and strong solubility emerges.
Technical Specifications & Labeling
Manufacturers provide the compound under strict quality controls, reporting minimum purity above 98%. Typical labels outline cation and anion identity, water content under 0.2%, and specific gravity values verified by density meters. Some suppliers include NMR traces as proof of structure, plus certificates of analysis so users know exactly what they’re getting. I’ve come across labels that spell out precise preparation date, shelf life, and safety warnings about exposure limits. Regular lots range from 25 grams to multi-kilogram containers, and temperature stability ratings alongside the UN hazardous goods code offer transparency for global shipping practices.
Preparation Method
Most labs begin synthesis with 1-butylpyridine and methyl iodide. These combine under reflux or in polar aprotic solvents, producing the quaternary methylpyridinium iodide salt. Next, the iodide cation meets lithium bis(trifluoromethanesulfonyl)imide in water so the metathesis dance can begin. Insoluble [BMPy][Tf2N] separates as a lower layer, which can be washed with water, dried under vacuum, and filtered to clarity. Those who work in bench-scale synthesis learn the pain of purifying ionic liquids—getting the last traces of halide out means repetitive washing, patient drying, and lots of waiting. Each step leaves a fingerprint on the product, which analysis by NMR, mass spectrometry, and Karl Fischer titration reveals.
Chemical Reactions & Modifications
Researchers constantly explore ways to tweak the pyridinium core, swapping in longer alkyl chains, adjusting the position or length of methyl groups, or replacing the [Tf2N] anion with alternatives like PF6-, BF4-, or dicyanamide. Simple alkylation reactions adjust the physical properties, often lowering viscosity or boosting ionic conductivity. The ionic liquid resists oxidation and hydrolysis under neutral conditions, but will decompose under strong acids or bases. Some work has looked into functionalizing the nitrogen on the pyridine ring, opening doors to covalent immobilization on surfaces or ion-exchange resins. The flexibility of this scaffold sets it apart from more rigid salts, giving it a head start in the race for custom-designed solvents.
Synonyms & Product Names
The nomenclature parade for this compound varies by source. You’ll spot it in catalogs as 1-butyl-4-methylpyridinium bis(trifluoromethylsulfonyl)imide, [BMPy][Tf2N], or plain old N-butyl-N-methylpyridinium bis(trifluoromethanesulfonyl)imide. Some chemical suppliers use CAS 79922-58-6 as a shorthand, crucial for regulatory tracking and procurement. People in the business quickly learn these alternative names, saving time on searches and avoiding confusion at the point of purchase.
Safety & Operational Standards
Working with this ionic liquid beats old-school solvents in terms of personal safety, but that doesn’t give anyone a free pass. Material safety data sheets warn users to avoid skin and eye contact, since the bis(trifluoromethanesulfonyl)imide group can irritate. Long hours in a glovebox or fume hood remain necessary for both purity and safety. Inhalation rarely poses a risk due to low volatility—an advantage that shifts the focus to accidental spills and chronic exposure. Emergency procedures revolve around standard lab practice: eye wash stations, chemical-resistant gloves, and proper ventilation. The industry eyes long-term toxicology closely, as the environmental fate of fluorinated anions becomes a bigger talking point. Responsible disposal involves specialized chemical waste streams instead of ordinary drains, echoing the growing movement around PFAS management.
Application Area
Fields putting this ionic liquid to work span energy storage, organic synthesis, separation science, and lubrication engineering. Laboratories testing lithium-ion and sodium-ion battery prototypes prefer [BMPy][Tf2N] as an electrolyte due to its broad voltage window and resistance to breakdown. I’ve had conversations with researchers exploring it in dye-sensitized solar cells, where low volatility trumps more hazardous mixtures. The oil and gas sector uses it as a specialty lubricant for extreme conditions—think valves and pumps that never see easy days. On the separation front, it forms the basis for green extraction of rare metals, pharmaceuticals, or even CO2 capture, since it swallows up a range of small molecules without releasing volatile organic emissions.
Research & Development
Academic papers and patent filings show this compound in the crosshairs of aggressive R&D around the world. Labs focus on finding ways to recycle it, boost its thermal stability, or combine it with nanomaterials for new hybrid systems. Battery research touches on constantly improving electrochemical stability and cycle life by tuning the cation-anion combination. Coordination chemists explore its ability to dissolve and stabilize transition metal complexes, which has a knock-on effect in catalysis and materials synthesis. Synthetic chemists invest time in seeing whether it can create more selective reaction profiles, reducing side product formation. Even teams looking at membrane separations see promise, grafting ionic liquids onto polymer frameworks to cut down on fouling or to tailor selectivity.
Toxicity Research
Ionic liquids proudly carry the green chemistry torch, though the truth about toxicity hinges on each structure’s quirks. Available studies, including cell culture and aquatic organism exposure, show [BMPy][Tf2N] exerts moderate toxicity in high concentrations, primarily due to the fluorinated anion. Chronic studies remain limited, but researchers stress careful handling and rigorous waste procedures, since bis(trifluoromethanesulfonyl)imide persists in environments. Ongoing efforts look at biodegradable alternatives, while ecotoxicologists push for a deeper understanding of lifetime environmental accumulation. Manufacturers face mounting pressure to divulge toxicity and life cycle impact, shaping policy and buying decisions well beyond the research bench.
Future Prospects
Everything points toward continued expansion for 1-butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide applications. If industries find reliable methods to recycle or degrade the anion, hesitations about PFAS-related persistence should shrink. Engineers and chemists appreciate how it can solve real-world problems across energy, materials, and environmental sectors, driving patents and process improvements. Future research may lean on AI to design next-gen variants with lower toxicity, faster kinetics, or even responsiveness to light or magnetic fields. My work with start-ups in green chemistry has revealed that well-characterized, versatile ionic liquids like this one bridge the gap between strict lab safety needs and aggressive industrial push for sustainability.
Opening the Door to Cleaner Chemistry
Labs looking for safer, eco-friendly ways to replace traditional organic solvents keep finding themselves circling back to ionic liquids like 1-butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide. This complicated name boils down to a class of compounds that doesn’t easily evaporate or catch fire. Unlike the cocktails of solvents found in older protocols, these salts often deliver the same performance with less environmental baggage. Back in graduate school, curious hands reaching for these bottles marked a quiet but steady step away from noxious fumes and hazardous waste.
Electrochemical Devices and Batteries
Electrochemistry circles have a lot to say about this compound because it offers high thermal stability and can handle wide voltage ranges without breaking down. That gets lithium battery developers excited. Researchers want electrolytes that don’t catch fire and can carry ions smoothly between the anode and cathode. When a doctoral colleague tested early blends containing this ionic liquid, safety results improved compared to carbonate-based mixtures. We all felt a bit more comfortable sticking around for the test runs. Beyond lithium-ion designs, supercapacitors also move closer to commercial reality as developers blend these ionic liquids for higher energy density and longer shelf life.
Catalysis
Catalytic reactions bring out some of the best features of modern ionic liquids. During a summer internship at a fine chemicals manufacturer, the technical team faced a stubborn reaction that needed low volatility and selective solvation. 1-butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide dissolved everything we threw at it—including tough metal catalysts—without setting off alarms for lost yield or dangerous conditions. Years later, turnover tests still show conversions bump up, and runoff waste becomes easier to treat.
Separation and Extraction
Many industrial processes grind to a halt when time comes to pick out a rare metal or separate similar organic compounds. Ionic liquids do not show the same limitations as water or chloroform. Because of their tunable structures, big mining operations and high-performance labs shape ionic liquids for rare earth extractions and gas separation. A few years ago, a process chemist I know ran trials on recovering palladium, and recovery rates shot up using this specific ionic liquid blend with almost no cross-contamination. Plant operators saved money, and environmental managers had fewer headaches from hazardous media.
Green Solvents for Synthesis
The green chemistry movement keeps growing because people want fewer hazardous solvents around workers. Regulations hit hard, and fines stack up when spills happen. In routine benchtop synthesis, swapping out volatile organic solvents for 1-butyl-4-methylpyridinium bis(trifluoromethanesulfonyl)imide simplifies compliance and waste disposal. Benchtop runs in teaching labs show students what progress looks like: fewer masks, open windows, and a sense that science can be both smart and safe.
Supporting Research and Future Directions
Colleagues in academia watch patents and papers pile up as more industries catch on to the flexibility in synthesis, electrochemistry, and extraction. Each application, from greener pharmaceutical production to safer energy storage, stacks up real-world benefits. Research into degradation products and recyclability will close the loop between excitement in the lab and smart rollout in factories. Scientists and regulators who lived through the era of benzene stains know that practical change comes one small bottle at a time.
The Real-World Stakes of Stability
Chemical stability often gets discussed in research labs, but its impact stretches further than test tubes and data charts. It shapes how medicines work, influences product recalls, and can tip the balance between safety and risk. Imagine grabbing painkillers for a headache, only to discover ineffective tablets months before their expiration date. That’s far from rare. Instability in compounds has led to wasted drugs, increased costs, and, much worse, patient harm. The World Health Organization highlighted this when it listed substandard or degraded medicines among leading threats to public health.
Daily Life Experience: Expired Products and Hidden Risks
Few people check expiration dates on cleaning products in the back of a closet. Yet, unstable compounds in household goods can lose their effectiveness, or even turn toxic. Bleach, for example, loses potency in sunlight or heat, dropping in effectiveness long before the date on the label. Once, I used expired hydrogen peroxide on a cut. It fizzed half-heartedly and failed to keep things clean. That mistake caused a minor infection—a small event, but a clear reminder that shelf life is not just a number for compliance; it guides actual outcomes.
Science Behind Stability
Let’s bring the conversation to the science behind it. Compounds break down due to factors like temperature, humidity, light, and even oxygen exposure. Researchers use accelerated stability testing, storing samples at higher temperatures and humidity, to predict how long a batch will last under real storage conditions. The United States Pharmacopeia sets guidelines for stability testing, followed by industries around the world. Ignoring these rules can mean products react with packaging, lose potency, or trigger allergic reactions due to new byproducts.
The Importance for Trust and Safety
No brand survives repeated recalls or routine failures. Pharmaceutical and food industries have earned trust, not through advertising, but consistent delivery of reliable goods. Incidents like the 2012 statin recall, where drugs broke down in humid climates, showed the real cost of ignoring shelf life. Patients ended up with less potent medication, setting back their treatment and causing immense stress. Public trust takes hits like this seriously, and regulators have tightened controls around chemical stability since.
Transparency and Better Practices
Clear labeling, better storage guidance, and smarter packaging all help. Foil blister packs, brown glass bottles, and silica gel packs routinely protect chemicals from degrading factors. Some companies use indicators that change color when exposure threatens quality. In my experience as a regular consumer and a researcher, these improvements have made it easier to trust the medicine or vitamins in my cabinet. Real progress comes from companies sharing stability data openly—providing batch numbers, test results, even QR codes that let buyers verify the actual testing behind their bottle.
Looking Toward Solutions
Chemists and industry leaders keep seeking new stabilizers, smarter polymers for packaging, and better coatings. Education helps too—reminding people that a storage cupboard isn’t always cool or dry, and that sunlight through a bathroom window works against shelf life. Tighter regulation also spurs honest labeling and discourages shortcuts. From my own mishaps and from countless stories reported in journals and news, two things stand out: chemical stability shapes the risk and reward of any compound, and shelf life should never be taken for granted.
Storing Materials: The Importance of Getting it Right
Keeping things safe starts with how we store what we use. I've worked in shops, kitchens, and garages, each with its blend of risks and messes. Strong safety follows from simple habits. For anything with even a whiff of danger—paint thinners, bleach, batteries, fertilizer—it goes on a shelf, not on the floor. Higher shelves keep leaks from spreading. Each bottle or bag faces outward so labels stay visible. I never tear off instructions; they matter on the day something goes wrong.
Certain products need airtight lids. Even tools that seem harmless, like solvents and oils, spoil or react with air. I've seen the mess left behind when folks forget to tighten a bottle cap and a week later, there's a sticky puddle at the back of a cabinet. A cleanup like that makes the case for careful storage better than any sign on the wall.
Temperature and Environment Matter
People often ignore warnings about heat, but a hot car trunk can turn spray paint into a hazard or cause battery packs to leak. Growing up in Texas, I learned the hard way that cardboard boxes melt into the floor of a hot shed. Moving everything into sealed bins kept the mice out and stopped smells from getting everywhere, especially during our humid summers.
Humidity harms a surprising range of products. Salt cakes together, flour spoils, even cleaning powders clump and lose effectiveness. I once left a bag of cement mix on a garage floor—after a rainstorm, the bag weighed twice as much and had to be thrown out. Dry places make for safer storage, and raised shelves work better than concrete floors, especially in any spot prone to water.
Handling: Simple Steps That Save Trouble
Handling starts with reading. For unfamiliar chemicals, I always check the directions. If the label has hazard symbols, gloves and eye protection come out. In my early days on a landscaping crew, one guy poured a concentrate straight into a sprayer—by lunchtime, his skin broke out in a rash. Our boss kept a binder of safety datasheets, and we learned to check it before using anything new.
Spills happen, even for the careful. I use rags for small spills, not paper towels that fall apart. Never try to mask strong smells with air freshener; ventilation clears the air fastest. Storing absorbent powder or kitty litter near cleaning supplies can help with bigger leaks, especially in workshops or garages.
Managing Risks at Home and Work
Homes often lack the rules seen at job sites, but risks stick around. Lockboxes or cabinets with childproof latches keep cleaners, medicines, and anything sharp out of reach. For folks with pets or kids around, remember: bright colors and fancy packaging attract curiosity. I always label containers I transfer products into, even if it feels redundant. A moment of confusion could bring disaster.
Sharing safe habits across family, friends, and coworkers sets everyone up for success. Safety isn’t about giant rulebooks but attention to detail. I’ve learned most mistakes come from skipping these steps under pressure—rushing through clean-up, moving supplies late at night, or storing something in the wrong place. A few extra minutes for care usually saves hours—or worse—down the road.
Why Melting Point Matters
Melting point helps you pin down how a substance behaves under heat. Ice melts at 0°C, table salt at 801°C. That tells us ice belongs in the freezer, while salt stays solid in a hot pan. Tracing back to my high school chemistry class, I remember testing unknown powders by heating them up. Fast changes—like sugar caramelizing on the stove—made it easy to spot what was in front of me. Any cook or metalworker knows to respect those crucial changes where a solid turns to liquid.
Stable drugs in pharmacy shelves depend on melting point, too. Medications that melt at lower temperatures risk turning sticky on warm days, losing shape or mixing badly. In food factories, chocolate-makers pay close attention so the final product keeps its shine without melting at room temperature. The right melting point separates a chocolate bar that stands up to the sun from a mess in your bag.
Solubility: More Than Just Mixing
Solubility measures how well something dissolves in a liquid. Table sugar scoops right into coffee and disappears, while sand sinks straight to the bottom. None of this feels abstract—anyone who's watched salt dissolve in soup understands solubility firsthand. In my early gardening tries, I once used Epsom salt to “heal” wilting plants; dissolved Epsom salt helped roots more than lumpy clumps ever could.
Medicine leans heavily on solubility. Doctors count on pills dissolving in the stomach, delivering medicine right to the bloodstream. If a drug resists dissolving, the body can’t absorb it well, leaving you paying for medicine that mostly leaves your system unused. Chemists spend months developing new drugs that dissolve only where needed—sometimes that calls for coatings or adding special sugars to the formula.
Water pollution catches many by surprise here. Some chemicals, like heavy metals or pesticides, dissolve and slip quietly into rivers, making cleanup tough. On the other hand, oily spills often float or clump, easier to skim off the surface. Floods and groundwater issues hang on subtle changes in solubility, a fact not lost on people living near old factories or farms.
Real-World Impacts and Common Sense Solutions
Anybody who’s tried to clean a greasy pan understands that hot water and some soap usually beat cold water—a direct nod to solubility and melting point working hand in hand. In school cafeterias or commercial kitchens, correct washing comes down to getting detergents and temperatures right so grime lifts away. These decisions, small as they seem, echo through public health when they prevent foodborne illness.
Scientists in labs and kids in home kitchens learn to respect these simple rules. Labs store volatile chemicals below their melting or boiling points for safety. Chefs temper chocolate to a narrow temperature window. Public water departments rely on both properties to choose how and where to add treatment chemicals safely and efficiently.
Teaching students through hands-on experiments, not just chalkboard notes, turns these facts into lessons that stick for life. Watching a salt crystal vanish or a block of ice disappear means more than reading a chart ever could.
Takeaway: Melting point and solubility aren’t textbook trivia. They show up in your cup of tea, your medicine cabinet, and the water that comes from your tap. Pay attention to them, and you’ll spot a thousand everyday decisions that hinge on these two simple facts.
Understanding Chemical Reactions Up Close
Working in a lab or running a process line, one of the very first questions that pops up before starting a new batch often centers on compatibility. It's more than ticking boxes on a safety sheet. Chemical mishaps don’t only threaten production—they can push serious safety risks onto teams, damage equipment, and generate lots of waste. That kind of aftermath isn’t just costly in dollars but in trust too.
Why Compatibility Actually Matters on the Floor
Over the years, I’ve seen what happens when teams assume two substances can mix without issue. Sometimes, nothing happens—until things heat up or someone changes a parameter, and then the reaction derails. At one facility, a poorly considered solvent swap ended up corroding a stainless steel vat that had served the site for years. Nobody realized that a certain cleaning agent would react with the new solvent and trigger pinhole leaks. The fix required not just swapping back the solvent but also gutting the tank and repairing entire sections of the line.
That story stuck, partly because it’s easy to fall back on habits from past jobs or take supplier guidance at face value. But as science and industry both become more complex, old approaches don’t cut it anymore. A minor change—a new thinner for a paint, a tweak in pharmaceutical excipients, or even cleaning out residue between runs—may trigger reactions no one expected.
How to Figure Out Real Compatibility
Experience shapes my approach, but data always leads. Reference charts from trusted sources like CDC’s NIOSH, the ACS, or big chemical suppliers remain go-to tools on the bench. They flag obvious risks: acids with bases, oxidizers near organics, etc. But not everything lives in charts. With more specialty solvents and new blends showing up every year, what worked in 1995 sometimes holds no water today.
I’ve watched colleagues get deep into technical manuals, but others prefer quick-and-dirty bench tests or tightly monitored pilot runs. Simple tactics—glass jars for spot tests, dipping metal coupons, or running small reactions under good ventilation—catch problems early. I trust those more than broad platitudes.
Risks and the Bigger Picture
Ignoring compatibility doesn’t just mean ruined batches. Take a flammable solvent mixed with an unstable chemical: static or heat could trigger a fire, even an explosion. Hidden incompatibilities can also lead to off-gassing, forming hazardous byproducts like phosgene or ammonia in small but dangerous amounts. In regulated industries—paint, food, pharmaceuticals, and medical—the fallout could mean product recalls, lawsuits, and regulatory intervention.
Real-world cases are full of sharp reminders: companies fined for environmental releases, or cleanroom contamination that wiped out multimillion-dollar yields. Even small shops feel the pain: customer trust disappears after a single faulty or contaminated batch.
Smarter Solutions in Daily Practice
Smart process managers regularly review compatibility before rolling out new chemicals or altering steps. Look beyond glossy datasheets: tap into supplier technical teams, peer-reviewed literature, or third-party safety auditors. Pull up material safety data sheets (MSDS), and make a habit of comparing them actively—not just filing them for insurance.
People matter too. Staff with good training spot trouble before it blows up. Regular refresher courses, actual hands-on drills, and open reporting make near-misses teachable instead of regrettable. A team that feels responsible skips assumptions and starts discussions, whether it’s about a novel green solvent or rethinking a cleaning routine.
Modern industry can’t depend on luck. Fast development cycles, tight tolerances, and regulatory scrutiny mean compatibility is not a footnote—it’s a core step that separates safe, reliable production from disaster.