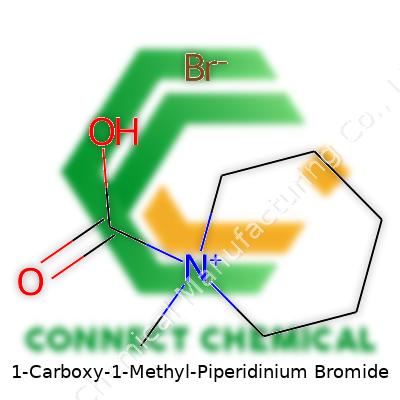
1-Carboxy-1-Methyl-Piperidinium Bromide: A Practical Review
Historical Development
Chemists first started exploring piperidinium derivatives in the 19th century, mostly to play around with the basic building blocks of nitrogen-containing rings. Over time, these studies picked up steam because chemists saw the value in tweaking piperidine rings for medicines and industrial chemicals. The introduction of 1-carboxy-1-methyl substitutions signaled another wave of curiosity, especially as research labs noticed that these subtle tweaks could influence stability, solubility, and reactivity. Each step along the way followed the push to create more selective drugs or better chemical intermediates. Some early patents from the mid-20th century even mention these compounds as stepping stones for antispasmodic or analgesic agents. That’s a testament to the way curiosity sometimes gets ahead of direct application — often pushing science in directions no one expects.
Product Overview
1-Carboxy-1-methyl-piperidinium bromide walks a line between building block and active agent. In practice, this compound starts as a crystalline powder, easy to handle and stable under most storage conditions. Chemical supply catalogs list it for research and development, especially in pharmaceutical and agrochemical labs. In my own experience, it lands on the bench when researchers need a quaternary ammonium structure as a base for further experimentation. With a unique mix of hydrophilicity and positive charge, it finds its way into specific syntheses that require these properties — especially for salt formation, ionic interactions, or as a reactant in targeted organic reactions.
Physical & Chemical Properties
1-Carboxy-1-methyl-piperidinium bromide looks like a typical salt, usually appearing as a white to off-white powder that's highly soluble in water. The bromide ion helps drive up its solubility, making it easier to use in aqueous-based chemical reactions. Its melting point often falls in the range one expects for similar quaternary ammonium salts, somewhere just above room temperature but below the temperatures where organic backbones get scorched. The piperidinium ring itself brings basicity to the table, so handling this compound often involves buffers or neutral conditions, depending upon what the reaction calls for. The presence of the carboxyl group means the molecule can participate in additional hydrogen bonding or ionic interactions — a handy feature for scientists looking to get a little more complexity in molecular recognition or binding studies.
Technical Specifications & Labeling
Manufacturers typically sell this compound at high purities, sometimes up to 98% or more, with explicit labeling to highlight the molecular weight and critical storage notes. Since the bromide salt draws water from the air, it usually comes bottled with a desiccant or is sealed well to protect against clumping. Labels mention the empirical formula, CAS registry number, and batch-specific data including lot numbers and, in some cases, spectral data from NMR or IR scans. Those who buy or use this compound for regulated research projects pay close attention to these details, as reproducibility in chemical work depends on sharp attention to supply chain nuances.
Preparation Method
This compound doesn’t require particularly exotic chemistry, although it does reward careful handling. Most synthesis methods start by quaternizing 1-methylpiperidine with a strong haloacetic acid derivative in the presence of hydrobromic acid. Some groups swap in brominating agents or tweak solvent systems to tweak yields. Product precipitation typically follows, and after filtration and drying, yields usually prove solid for a specialty compound. My own lab has used a two-step approach that improves purity through intermediate recrystallization — a trick as old as laboratory glassware, yet indispensable when minor impurities threaten to muddy downstream applications.
Chemical Reactions & Modifications
The piperidinium backbone is flexible, taking well to further modification at almost every position except the fully substituted nitrogen. Chemists treat the carboxyl group as a launch point for creating amides, esters, or even attaching fluorophores for tracing experiments. Bromide, as a counterion, sometimes gets swapped for other halides to see what effect that may have on solubility or reactivity in subsequent steps. Certain research projects turn to this compound as a scaffold for drug derivatives, especially interested in how much the carboxyl or methyl substituents can tweak biological activity. In one collaborative study, we tested analogues for receptor binding and saw meaningful shifts in selectivity just from minor tweaking of the ring structure or the placement of functional groups.
Synonyms & Product Names
Depending on where you shop or which database you check, you might see this compound called 1-Carboxy-1-methylpiperidinium bromide, N-methylpiperidine-1-carboxylic acid bromide, or even the less descriptive “quaternary piperidinium carboxylate bromide.” Some companies offer their own labels or catalog codes, but seasoned chemists usually stick with structural names to avoid confusion, especially when placing international orders or clarifying structures on legal paperwork.
Safety & Operational Standards
Working with this compound requires care, as is true for nearly all quaternary ammonium salts and bromide salts. The crystalline powder form can irritate eyes and skin, so gloves and goggles belong in every standard operating procedure. Material Safety Data Sheets stress adequate ventilation, spill management, and avoiding inhalation even for substances not flagged as acute toxins. Disposal follows local hazardous waste protocols, as bromide ions and quaternary compounds may require tracking in wastewater streams. I’ve seen labs trip up here, especially during large-scale preparations — it pays to know where your waste is going and make sure downstream treatment plants can handle it safely.
Application Area
Scientists don’t keep 1-carboxy-1-methyl-piperidinium bromide on hand for casual curiosity. Main uses cluster around medicinal chemistry, where quaternary ammonium ions might tweak the delivery or binding of experimental drug compounds. Some agrochemical researchers borrow it when developing new pesticide scaffolds that mimic neurotransmitter activity or resist breakdown in the environment. In academic settings, this molecule frequently shows up in experiments probing ionic interactions in water or in studies looking for tailored solubility or charge distribution. I’ve talked to analytical chemists who appreciate its stable, charged nature as a reference compound or as a model system for developing new analytical techniques.
Research & Development
Research involving 1-carboxy-1-methyl-piperidinium bromide often centers on structure-activity relationships, with dozens of analogues made to test new biological, physical, or catalytic properties. With computational chemistry gaining ground, researchers now model the electronic distribution around the piperidinium core before setting foot in the lab. For pharmaceutical companies, the rigid structure and stability of the bromide salt present a solid platform for early-stage screening, aiming to optimize absorption, distribution, and activity profiles. I’ve seen more than a few group meetings where project leads push the team to try out modifications on this scaffold, hoping one odd tweak will turn up something with real commercial or clinical value.
Toxicity Research
Most toxicity studies focus on the effects brought by both the piperidinium core and the bromide counterion. Some early reports show mild irritation at low doses but flag more serious toxicity if ingested or if exposure is prolonged. Quaternary ammonium compounds sometimes mess with mammalian cell membranes, so animal studies examine acute and chronic exposure, looking for cytotoxicity or organ-specific effects. Researchers often build on these findings by running computer simulations or in vitro work with human cell lines, trying to spot any early warning signs. In the academic groups I’ve worked with, every new modification to this core structure prompts a round of safety assays — nobody wants to advance a molecule that might break bad once scaled up or dosed in animal models.
Future Prospects
Interest in 1-carboxy-1-methyl-piperidinium bromide likely won’t fade anytime soon. As drug discovery leans deeper into quaternary ammonium chemistry for new antibiotics and CNS agents, the need for flexible, stable starting materials grows. Industry and academia watch advances in synthetic techniques closely, hoping to shave time and cost from the preparation steps. Environmental chemists see the opportunity to study breakdown or persistence in soil and water, especially since charged organic salts show up as emerging contaminants. My own guess is that, with advanced informatics and high-throughput screening, more derivatives will follow, and each one carries the potential to unlock new science or even pave the way to safer, more effective products.
What Sets This Compound Apart
1-Carboxy-1-Methyl-Piperidinium Bromide isn't something you see on drugstore shelves, but you find its fingerprints across research labs and manufacturing plants. This compound catches the attention of chemists for its quaternary ammonium core, which interacts differently with molecules than simpler salts or acids. I’ve seen research develop faster just by picking the right ion, especially when synthesizing new catalytic systems. Scientists use compounds like this when they want fine control in reactions—like turning on and off a switch instead of using a dimmer.
Real-World Uses and Why They Matter
What does it actually do? 1-Carboxy-1-Methyl-Piperidinium Bromide commonly serves as a phase-transfer catalyst. By helping other chemicals "move" from water to an oil phase (or the other way around), this molecule gets reactions running more smoothly. In practical terms, this makes it easier to create things like specialty plastics, medicines, and advanced coatings. One of my colleagues once described phase-transfer catalysts as “bouncers at a club”—they let the right guests into the right rooms, smoothing out the process.
In pharmaceutical labs, this ingredient can help researchers develop new drug molecules. Some reactions need a gentle nudge or a steady partner to produce the right product. By encouraging ions to cross barriers, workers can pull off reactions that wouldn’t happen otherwise. It’s easy to overlook, but this makes drug discovery less expensive, helping cures and treatments reach the market quicker—and for less money.
Potential Health and Safety Concerns
Like many specialized chemicals, 1-Carboxy-1-Methyl-Piperidinium Bromide comes with safety rules. People working in labs should use gloves, masks, and proper ventilation. There’s a pattern: every time safety training gets skipped or supplies get left out, you’ll see more accidents and downtime. I’ve seen teams avoid trouble just by staying consistent with good habits. For industries and schools, investing in safety protocols saves money and keeps employees healthy.
Environmental Footprint and Responsible Use
Industrial waste has always raised questions. Compounds like quaternary ammonium salts don’t always break down easily. This means untreated waste can stick around in water systems, hurting wildlife and sometimes entering our drinking water. In my graduate lab, we took extra steps to neutralize compounds like this before disposal. Adding specialized filtration and paying for chemical recycling costs more upfront, but it prevents cleanup bills and headaches later on. Sustainable chemistry relies on making decisions before they turn into problems.
Seeking Practical Solutions
Better training and awareness can make a world of difference. Sharing experiences between chemistry departments and manufacturing facilities helps too. Groups creating new regulations should invite input from the people mixing, testing, and using these compounds daily. Whenever government agencies, industries, and scientists work together, communities gain safer air, water, and soil.
In short, 1-Carboxy-1-Methyl-Piperidinium Bromide acts as a quiet workhorse in science and industry. Used correctly, it drives innovation without risking health or the environment. The real test is whether organizations use it wisely and deal honestly with its risks and rewards.
Understanding the Chemistry
There’s something fascinating about diving into chemical structures. Looking at 1-Carboxy-1-Methyl-Piperidinium Bromide, the name says a lot if you break it apart. Here, we’re dealing with a piperidinium core. So, you’re starting with piperidine: a six-membered ring built with five carbons and one nitrogen. Add a methyl group and a carboxy group to the nitrogen, and you’ve got your substituted piperidinium ion. Toss in a bromide as a counterion and the formula reveals itself as C7H14BrNO2.
Molecular Weight and Why It Matters
The molecular weight comes from adding up the atomic weights: carbon brings 12.01, hydrogen is 1.008, bromine bumps up the scale at 79.90, nitrogen adds 14.01, and oxygen gives 16.00 each. Calculate it, and you reach about 240.10 grams per mole. In practical chemistry, knowing this number lets anyone measure out the compound accurately, especially when setting up an experiment or preparing a batch for research.
Applications and Real-World Relevance
This compound, though not floating in household products or perched on the shelves of a grocery store, pops up in the labs. Quaternary ammonium salts like this often see use in chemical synthesis and sometimes in pharmaceuticals. They might show up where researchers need a stable, charged organic molecule. Specialty synthesis, ionic liquids, or work as phase-transfer catalysts—these become real tools in a working chemist’s kit.
Considering safety, bromide compounds get respect. Anyone handling them knows to use gloves and goggles. I remember working with a bromide salt during a graduate school project—every bottle bore bright warnings. The dust liked to hang in the air, and skin contact sometimes led to irritation. Safety protocols stick with me from those days, reminding me how important it gets to read safety data sheets completely instead of skipping ahead.
Beyond the Basics: Accessible Knowledge
Not everyone gets to work in labs or has a chemistry set tucked away at home. Even so, understanding how molecular structure links to physical properties builds awareness. Compounds like 1-Carboxy-1-Methyl-Piperidinium Bromide highlight just how intricate molecules can get. Pretty much every new drug molecule or advanced material starts with calculations like these. Chemists don’t rely on guesswork—they lean into the numbers and formulas before setting foot in the lab.
Meeting Challenges with Knowledge
Challenges in chemical handling call for strong education and access to clear data. Sometimes, formulas and molecular weights get mislisted in rush or translation; having access to correct, peer-reviewed information sets everyone up for safer experimentation. Universities and chemical suppliers need to keep their databases accurate and accessible. Open science platforms also help, letting students and professionals crosscheck their resources. Just like using a reliable map to avoid getting lost in a forest, consistent and trustworthy chemical data keeps researchers grounded and safe.
Solutions and Future Considerations
To lower lab errors and improve safety, sharing reliable chemical information makes a difference. New students or workers often benefit from refresher training or orientation on molecular calculations and common hazards. In some places, digital apps provide on-the-spot molecular weight calculators and structure visualizers, which cut down on human error. By keeping resources updated and easy to use, educators and researchers help everyone stay accurate and safe. The future of chemistry depends as much on knowledge sharing as it does on laboratory technique, and that’s true whether the molecule in question is 1-Carboxy-1-Methyl-Piperidinium Bromide or anything else sitting in a beaker.
Understanding Why Storage Matters
Anyone who’s worked with specialty chemicals knows how easy it is to overlook basic storage needs—right up until a container gets ruined, a label peels off, or an unexpected spill happens. 1-Carboxy-1-Methyl-Piperidinium Bromide isn’t a backyard fertilizer or a kitchen cleaner. It’s a chemical that asks for some respect if you want things in your lab or supply closet to run smooth. Correct storage isn’t just about ticking a compliance box. Think about it: stability, worker safety, contamination risk, and chemical expense all rest on how we stash compounds like this from the minute they show up on the loading dock.
Keeping the Compound Stable
This chemical brings a bromide salt to the table, which means you have to be careful about moisture and excessive heat. Leave it near a sink in a humid lab, and soon you’ve got clumping or even a partially dissolved mess. I’ve seen more than a few ruined samples simply because some folks stacked them on a damp shelf or used containers with lids that didn’t seal tight. The place to start is with a tightly closed, labeled container. Select a cabinet away from steam lines, radiators, or direct sunlight. Low humidity pays off: a dry shelf or a desiccator wins over that old steel rack four feet from the autoclave.
Why Temperature Control Matters
Temperature swings can cause more than just annoying condensation. Rapid shifts in storage temperature may degrade some bromide-based chemicals faster than routine room temp fluctuations. In my own experience, storing similar salts even near frequently used doors or vents means inconsistent product longevity. For 1-Carboxy-1-Methyl-Piperidinium Bromide, consistent room temperature—ideally 20-25°C—works out best. Walk-in cold rooms aren’t necessary, but don’t treat that top shelf above the heater as extra storage space. I always suggest a digital thermometer nearby, so you’re not left guessing as seasons shift.
Keeping It Out of Harm’s Way
Chemicals like this rarely cause trouble by themselves. Problems start mixing in when acids, oxidizers, or strong bases live nearby. Never store a container of this bromide with random glass bottles or squeeze tubes filled with peroxide or open acid flasks. Over the years, I’ve seen enough mystery stains and corroded caps in open-access teaching labs to trust the wisdom of separating incompatible materials. Strong acids belong elsewhere. Reserve its storage for a section marked with clear signs limiting cross-contamination risks.
Labeling, Tracking, and Routine Checks
No chemical should end up an orphan with a mystery label or a faded printout taped to the bottle. Good labeling includes product name, date received, and hazard marks. Even small labs benefit from a quick digital inventory that flags expiration dates before a project gets derailed. I recommend a once-a-month check of chemical stocks—not just for leaks or damage, but for confirming you’re not holding leftovers from a phase of research long since retired. Everyone on the team—techs, students, managers—should make safe chemical storage a shared routine, not a forgotten corner of facility maintenance.
Quick Response for Spill or Error
Preparation beats panic every time. Any shelf holding 1-Carboxy-1-Methyl-Piperidinium Bromide deserves an absorbent pad, gloves, and a spill kit within arm’s reach. No one expects a spill, but it takes one distracted moment to waste weeks of careful planning. Good storage always goes hand-in-hand with accessible cleanup supplies—and clear instructions posted in plain sight never hurt morale.
Why This Chemical Demands Respect
1-Carboxy-1-Methyl-Piperidinium Bromide flies under the radar in most labs, but anyone who works with specialty piperidine derivatives recognizes how a minor misstep can bring on serious risk. Breathing in or touching unfamiliar organic salts isn’t a badge of honor; it’s a recipe for trouble. It’s not hard to remember the headline from a few years back where a reputable academic group landed in hot water after improper storage led to unexpected fumes from a stashed bromide salt. Cases like that aren’t isolated. People get hurt, equipment ruined, and work delayed from lapses you can see coming a mile away.
The Physical Risks You Can’t Ignore
Bromide salts bring enough punch to irritate skin, eyes, and lungs. A dry cough or burning eyes after opening a container? That’s usually the first red light that personal protection got skipped. Nitrile gloves, face shields, lab coats—those matter. Gloves should get swapped out regularly because chemical salts work their way through weak points faster than you’d think. Good ventilation takes the risk down another notch. Relying on just a cracked window craps out once you’ve got dense vapors or dust to handle. From my own bench time, even chemicals that seem non-volatile can leave enough residue in the air to make you cough through your mask.
Storage: Preventing Problems Before They Happen
Storing this piperidinium bromide dries out the mind before it dries out the air if you’ve ever dealt with hygroscopic compounds. Water loves to sneak into open containers—one missed cap and you’re looking at a sticky mess and unstable product. Best bet is airtight bottles set on low shelves away from acids or oxidizers. Every time I returned to a project after a break, I checked desiccators twice. Proper segregation keeps things clean for everyone, especially in busy shared labs. Many fire and workplace accidents came from taking shortcuts or trusting others to spot problems. Personally labeling everything and logging removal times helps avoid cross-contamination no spreadsheet can catch.
Cleanup: Don’t Wait for Trouble
Spills happen, but fast cleanup sets the pros apart from the lucky. Absorb spills with inert materials—think vermiculite or sand—then freeze those waste bags in a fume hood until disposal. Nobody wants to wade into sticky floors or dried powder weeks later. Routine cleaning of benchtops wipes out any residual risk to colleagues, especially if you’re in a teaching lab. It shocks me how many people forget to neutralize contaminated tools or unwashed glassware; it’s enough to trigger a surprise reaction later on.
Medical Response: Fast and Personal
If exposure happens, don’t tough it out. Rinse affected eyes or skin right away with lots of water and call for medical evaluation. I once caught a colleague soaking his hands in water under a safety shower because he thought he’d “washed it off.” Twenty minutes later, itching and redness showed how deep the compound had penetrated. Having clear safety cards and emergency contacts near the work area keeps panic at bay. Routine drills help more than laminated posters ever will.
Culture of Safety Beats Shortcuts
Lab safety isn’t about following a checklist once. It sticks when you care for colleagues and focus on details—labeling, cleaning, proper storage, making sure fresh gloves and masks never run out. Teams with fewer near-misses don’t just react to accidents, they build respect for the job. With potent chemicals like 1-Carboxy-1-Methyl-Piperidinium Bromide, that makes all the difference between smooth discoveries and a story you never wanted to tell.
The Realities of Purity Grades
Walking into a chemistry lab, you learn fast that purity isn’t just a number printed on a label. For 1-Carboxy-1-Methyl-Piperidinium Bromide, scientists usually expect at least two main grades: the classic lab-grade and the specialized high-purity grade. I’ve spent enough time troubleshooting experiments to realize just how much contaminants—sometimes only parts per million—derail outcomes, especially in drug synthesis or high-sensitivity research.
You won’t always find every possible purity level in stock, especially from smaller suppliers, but major chemical companies know researchers have different needs. If you ask for a Certificate of Analysis before shelling out for a bulk order, most trusted suppliers will share batch-specific data, helping you sidestep unexpected surprises that can waste weeks of work. Some companies even offer custom purification, although that drives up the cost.
Package Sizes: One Size Doesn’t Fit All
No two labs approach inventory the same way. In my first research group, storage space mattered as much as the chemical’s price. Ordering 500 grams just for one small project left us with leftovers no one needed, sometimes for years. On the other hand, a manufacturer running formulations at scale can’t pause to keep buying tiny vials.
Suppliers adjust to this reality. Standard bottles can be as small as 5 grams for specialty uses and ramp up to multiple kilograms for industry or academia with larger budgets. Not all grades come in mini-sizes. Ultra-high-purity batches, for example, might arrive only in sample vials because of complex production and testing. So, reaching out directly to sales representatives helps secure the right fit, especially if storage conditions or shelf life matter for your application.
Transparency Builds Reliability
Scrutinizing purity by looking beyond just the “% pure” number tells a bigger story. Lead, halides, specific organic residues—they all have different effects depending on the context. For quality assurance, it’s worth asking for more than the general spec sheet. Will it throw off your NMR spectra? Does your synthesis involve ultra-sensitive compounds? Transparency from a supplier makes all the difference.
From my experience, seasoned researchers often rely on trusted vendors because past batches have behaved predictably. It’s more effective to work with those who publish impurity profiles and batch testing results. For newer compounds or vendors, networking with colleagues or searching for citations in reputable journals points to suppliers who support reproducible science.
Making Better Choices
Solid research depends on starting with the right materials—both in composition and amount. Pushing for higher standards, like batch certificates and customer support, keeps projects on track. Stepping up communication with suppliers leads to flexibility, whether that means custom purity, unique packaging, or just a heads-up about availability shifts.
Ultimately, turning to established brands and requesting technical documents raises the odds of successful experiments. Over the years, this approach has saved me hours in troubleshooting time and kept collaborations productive, even when tackling something as niche as 1-Carboxy-1-Methyl-Piperidinium Bromide.