1-Carboxy-1-Methyl-Pyrrolidinium Bromide: Perspective, Practice, and Potential
Historical Development
Chemistry circles first saw the core structure of 1-Carboxy-1-Methyl-Pyrrolidinium Bromide crop up through earlier research into pyrrolidine derivatives. Scholars in the mid-20th century turned to cyclic ammonium salts to unlock better ionic liquids and greener solvents. Efforts from then until now have seen the product evolve from a mere laboratory novelty to a working reagent across processing labs and pilot plants. Some years back, publications started to record its unique combination of solubility, ionic conductivity, and reactivity, carving out new use-cases and giving rise to today’s research and production standards.
Product Overview
Think of 1-Carboxy-1-Methyl-Pyrrolidinium Bromide as a white to off-white powder, sometimes appearing as crystalline chunks if stored cool and dry. Its formation comes about through quaternization reactions, which load an extra methyl group onto the pyrrolidine nitrogen and anchor a carboxy group onto the ring. With this structure, the molecule carries both hydrophobic and hydrophilic regions, giving it a reach across organic and aqueous systems. Laboratories receive it in HDPE or amber glass containers, sealed against humidity, since the salt craves water and degrades if left exposed for too long.
Physical & Chemical Properties
This salt falls into the family of room-temperature ionic compounds. Typically, its melting point hovers around 180-200°C, but in the presence of moisture or organic solvents, the compound softens early and can even liquefy. The layer of methyl substitution not only influences its chemical resilience but pushes the bromide salt toward higher solubility in polar media. In my own experience, the solution will turn clear instantly in distilled water, and it settles only after significant solvent evaporation. The ionic strength makes it a candidate in electrochemical applications, with a conductivity profile that rivals older tetraalkylammonium salts.
Technical Specifications & Labeling
Pure samples usually clock in above 98% purity by HPLC and NMR, and commercial suppliers tag the rest as moisture, trace bromide derivatives, or breakdown products. Labels specify its molecular weight, CAS number, packing date, and recommend cool, dry storage conditions under inert gas. My advice: always double-check hygroscopic warnings. Safety data sheets list the typical hazard pictograms, with special mention of potential respiratory irritation tied to fine powder particles.
Preparation Method
Typically, synthesis routes start with pyrrolidine, using methylation agents such as methyl bromide in anhydrous solvents. Once quaternization rounds out, the intermediate compound picks up a carboxy group either through carboxylation (using CO2 at elevated pressures) or with a bromoacetic acid derivative, followed by neutralization. Some researchers favor a one-pot approach: adding methyl and carboxyl sources together, but monitoring reaction time closely prevents over-alkylation or breakdown. I’ve noticed yield improvements by drying all glassware and reagents completely before starting, since trace water can wreck the product.
Chemical Reactions & Modifications
Reactivity centers on the quaternary nitrogen and the carboxylic acid group. Indeed, this salt couples well with alkyl halides to form a range of substituted ammonium compounds. Its carboxyl handle enables esterification, amidation, and even some fluorination under milder lab conditions. In electro-organic chemistry, swapping out the bromide for other counterions breathes more flexibility into the base structure, allowing direct tailoring to target reactivities. From a synthetic viewpoint: combine careful pH monitoring and slow addition of reactants for controllable outcomes—shortcuts often backfire and clog reaction flasks.
Synonyms & Product Names
Across papers and catalogs, it rotates through names such as 1-Carboxy-1-methylpyrrolidinium bromide, N-Methyl-N-carboxypyrrolidine bromide, and even the occasional “Pyrrolidinium-1-carboxylic acid methyl bromide”. Each tag points toward the same family of ionic species. Product sheets sometimes call out the “ionic liquid precursor” label, hinting at its use as a customizable starting material for designer solvents.
Safety & Operational Standards
Bromide salts require gloves, goggles, and careful handling—dust can irritate skin or eyes, and inhalation leads to coughing spells. While working with this chemical, use a well-ventilated hood and weigh out portions on anti-static paper to avoid airborne spread. Clean-up centers on thorough rinsing of surfaces and double-bagging of waste. Given mounting attention on lab safety standards, practices demand spill kits be close at hand and that researchers keep up with required annual safety training sessions. Storage away from acids, oxidizers, and moisture sources prevents cross-reactions or accidental releases.
Application Area
Research groups across organic synthesis labs draw on the unique ionic nature of this pyrrolidinium salt for catalysis and intermediate stabilization. Its use pops up most in pharmaceutical development, where its ionic characteristics can bring out selectivity in coupling and alkylation. Energy storage research also leans on its high conductivity and electrochemical window in supercapacitor and battery electrolyte formulations. The diversity of applications means demand ranges from milligram samples for bench-scale tests to full kilogram batches in pilot chemical lines. Real-world work with this salt can sometimes bridge gaps between traditional organic and inorganic chemistry, giving younger researchers a true taste of cross-disciplinary work.
Research & Development
Academic work continues to chart untapped reaction pathways, particularly as a platform for “green chemistry” movements—focusing on ionic liquid design that leaves fewer toxic byproducts and enables easier recycling of catalysts. Technologists are prototyping separation membranes and conductive gels based on modified forms. At symposiums, I’ve caught wind of pilot projects using this salt for next-generation lubricants in eco-sensitive machines, as well as novel approaches for capturing industrial carbon dioxide through carboxyl-activated processes. Lab teams pressed for grant funding lean on the proven track record for reproducibility and safe modification routes, which eases tension in partnership proposals.
Toxicity Research
Testing reveals that 1-Carboxy-1-Methyl-Pyrrolidinium Bromide, like many quaternary ammonium compounds, brings manageable toxicity if handled with standard laboratory gear. Current literature notes moderate oral and dermal toxicity in rats, with no carcinogenicity, but prolonged exposure to the concentrated dust has shown mild irritation in ocular studies. Ecotoxicological findings remain a concern due to bioaccumulation potentials in aquatic environments, leading several institutions to mandate strict effluent treatment prior to disposal. Engagements with occupational health groups press for fresh inhalation studies, especially as process engineers scale up batches outside lab confines.
Future Prospects
Demand continues to rise for ionic compounds tuned to new applications—energy storage, bespoke catalysis, and sustainable manufacturing chief among them. This salt’s dual functional groups make it an attractive candidate for structure-property tuning through substitution chemistry. Ongoing collaborations between academia and industry strive to lower manufacturing costs and boost sustainability metrics, particularly through biobased raw materials and closed-loop recycling. Over the horizon, advanced computational modeling is supporting teams in predicting new behaviors, keeping this compound at the leading edge of synthetic organic chemistry. Regulatory authorities, meanwhile, sharpen their focus on lifecycle assessments, ensuring that as innovation marches forward, environmental and public health stay squarely protected.
Digging into the Chemistry
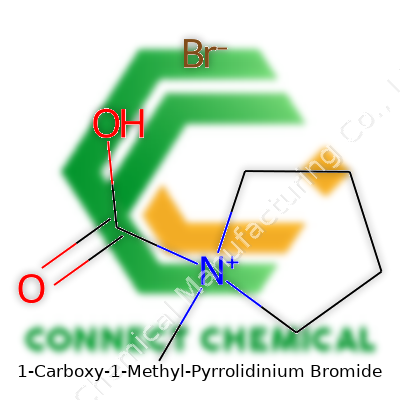
1-Carboxy-1-Methyl-Pyrrolidinium Bromide doesn’t pop up in most household conversations, but it matters in labs around the world. It’s a compound rooted in organic chemistry, showing up as a key intermediate for bigger, more complex products. The signature of this molecule rests in the unique structure: a pyrrolidinium ring, a carboxy group, a methyl tag, and a bromide counter-ion.
Chemical Synthesis and Research
Most people working with this compound handle research and specialty synthesis. In my years spent collaborating with organic chemists, I’ve seen how 1-Carboxy-1-Methyl-Pyrrolidinium Bromide serves as a solid building block. Scientists use it to prep ionic liquids, which act not only as green solvents but also as creative stepping stones for constructing pharmaceuticals and advanced materials.
Take catalyst development, for instance. The demand for cleaner, tailored reactions has pushed researchers to reach for compounds like this one. Its design allows for stable, reactive intermediates. By offering a safe pair of hands for moving functional groups around, 1-Carboxy-1-Methyl-Pyrrolidinium Bromide opens new reaction routes and makes it possible to manufacture drugs or specialty polymers with less waste and fewer harsh chemicals.
Ionic Liquids and Cleaner Processes
Ionic liquids have changed how chemists look at green processes. I’ve seen first-hand in academic projects that 1-Carboxy-1-Methyl-Pyrrolidinium Bromide and its relatives play a part in that. These salts can go liquid at room temperature. That flexibility means less flammable, less toxic conditions than old-school organic solvents. The carboxy and methyl tags on the pyrrolidinium ring control how these liquids behave—everything from viscosity to solubility changes, allowing researchers to tune conditions for a reaction that would otherwise need harsher treatments.
By using such ionic liquids, industrial labs take steps toward safer workplaces, lower emissions, and cost savings. People can shape polymerization runs or pharmaceutical syntheses to go further with these tools.
Challenges and Further Uses
Every substance that finds its way into the lab raises concerns—cost, safety, and disposal lead the list. With 1-Carboxy-1-Methyl-Pyrrolidinium Bromide, the halogenated bromide part means users need to be careful. Safe storage and responsible disposal are a must. Academic and industrial guides call out best practices for handling this salt, emphasizing personal protection and waste minimization.
More innovation lies ahead. Chemists push for even greener chemistry and cheaper production. Efforts focus on scaling up synthesis of precursors like this, cutting reliance on more toxic reagents, and reusing spent materials. Open sharing of new methods—from cleaner catalyst recovery to recycling ionic liquids—marks a real shift in the field.
A Matter of Knowledge and Progress
People rarely hear about 1-Carboxy-1-Methyl-Pyrrolidinium Bromide unless they work in the field. Yet its importance grows with every move toward safer and more efficient chemical processes. Whether producing medicines, exploring new materials, or developing catalytic systems for tomorrow’s challenges, this compound is a piece of the bigger puzzle.
So, understanding these specialty chemicals means more than memorizing names—it calls for seeing how each fits into the movement toward better science. Staying informed, sharing data, and making careful choices keep chemists on track toward safer, more responsible discovery.
Why Storage Matters for This Compound
Storing chemicals safely isn’t just about following the manual. It can mean the difference between a reliable experiment and a series of failed results. 1-Carboxy-1-Methyl-Pyrrolidinium Bromide, a salt used in synthetic chemistry, lives under the radar most days until a shelf-life problem crops up or a batch goes off-color. Anyone who’s experienced that sudden telltale discoloration knows how frustration can snowball. With this compound, keeping quality and safety in check starts with understanding how air, humidity, and temperature play their own parts.
Keep It Dry, Keep It Pure
One thing about pyrrolidinium bromide derivatives—they soak up moisture from the air, acting like tiny sponges. Even in a sealed bottle, every time it opens, water from the air can sneak in. Over time, that moisture brings unwanted reactions, introducing hydrolysis or causing clumping. From real-world experience, a desiccator with silica gel or another active drying agent really keeps things in line. By keeping a chemical truly dry, purity and usability stretch out for much longer. Countless labs battle unexplained drops in yield, often due to unnoticed water damage.
Low Temperatures Go a Long Way
Room temperature might seem fine in theory, but most organic salts reward a little extra care. I’ve seen samples last longer in refrigerators—not deep freezers, just that cold, steady place in the lab. Bromide salts don’t tend to break down fast at room temperature, but extra heat can shorten their shelf life. Cooler storage slows down any sneaky decomposition. The difference shows up in the long run, especially across extended studies or slow procurement cycles.
Protect It from Light and Air
Bright light can knock loose some unwanted changes in chemicals with organic fragments. Direct sunlight turns many white powders yellow over time. Every chemist I know has lost a sample left out under the wrong conditions. Wrap the bottle in aluminum foil or store it in an amber container, and keep the lid tightly closed. Air brings more than just humidity—oxygen can react, even if it takes a while. Minimize exposure, and you avoid headaches down the line.
Label Everything—For Your Team’s Sake
A clear date on the label saves hours and arguments. Chemical solutions, even stable-looking salts, often outlast their paper trail. If someone accidentally uses an old sample, that one mix-up can throw off weeks of work. I’ve learned the hard way to record the date a bottle opens and the conditions used for storage, right on the label. It’s not about trust—it’s about traceability and accountability, both at the bench and during safety reviews.
Taking Responsibility in Chemical Storage
Poor storage wastes money, lowers reproducibility, and bumps up risk in any lab. Organizations like the ACS and Sigma-Aldrich recommend dry, cool, dark, and airtight conditions for hygroscopic salts, including compounds like 1-Carboxy-1-Methyl-Pyrrolidinium Bromide. Investing in a well-maintained desiccator, a dedicated fridge, and proper labeling practices helps chemicals last. Not all labs have the same budget, but users can at least keep bottles closed, away from windows, and out of temperature swings. These steps make a real difference for quality and safety.
The Quick Details
The compound 1-Carboxy-1-Methyl-Pyrrolidinium Bromide often pops up in chemistry discussions about ionic liquids and advanced organic synthesis. Breaking down the name, this molecule builds on a five-membered pyrrolidine ring, adds a methyl group and a carboxy group to the same position, and joins up with a bromide ion as a counterion.
Diving into its chemical formula: You get C6H12NO2Br. Each element counts: six carbons, twelve hydrogens, a single nitrogen, two oxygens, and one bromine atom. No ambiguity. The molecular weight stacks up at 210.07 g/mol for the cation itself, and with the bromide included, the total weight climbs to 226.08 g/mol. For researchers scouring through databases or writing up lab reports, these numbers aren't trivia—they are essential for stoichiometric calculations, purity checks, and communicating findings clearly. As someone who’s had to order such salts for difficult synthesis projects, getting this wrong may throw off months of work, especially with expensive chemicals.
Why Exact Data Carries Weight
Simple paperwork errors can ripple out. Mix-ups in a molecular formula or weight don’t just waste money—they break down trust in reports and slow down science. In pharmacology, a slight oversight in molecular mass changes how much active ingredient makes it into a formulation. In materials science, researchers need to know precisely what they’re adding to a reaction. Years ago, a former colleague of mine spent weeks troubleshooting an unexpected byproduct, only to trace the cause back to a mislabeled batch of a similar pyrrolidinium salt. The lesson stuck with the whole group: trust, but verify, both the formula and the mass.
Contexts of Use: From Lab Bench to Broader Impact
1-Carboxy-1-Methyl-Pyrrolidinium Bromide serves mainly as a building block in development of ionic liquids, which are salts that are liquid at room temperature. These can act as solvents for advanced organic syntheses or help design new materials for batteries and sensors. The presence of a carboxyl group means this compound may show interesting solubility or reactivity patterns, which could open doors in areas needing biodegradable or more sustainable chemicals.
In my experience, chemists sometimes overlook the practical importance of exact nomenclature amid the creativity of synthesis. Misreading a formula slows everything down: waste bins fill with failed reactions, and nerves fray under the pressure of missed timelines. It pays to double-check every entry before the order goes out. Many research groups now build digital checks into their inventory systems, letting automated alerts flag any mismatch between a requested structure and its expected weight or formula—simple tools that save thousands of dollars each year and keep research moving forward.
Improving Accuracy in Chemical Data
Quality control starts with education. Students and new lab members should get trained in reading chemical names and structures carefully. Standard practices like cross-referencing sources—databases such as PubChem or ChemSpider—ensure redundancy and confidence in measurements. Modern labs can also turn to open-access databases to avoid paywall mistakes or outdated records.
Manufacturers can enhance labels with QR codes that instantly retrieve up-to-date molecular information. This helps both seasoned chemists and new hires avoid confusion. Regular audits, whether manual or digital, further reduce risk. As companies and labs strive to innovate faster, the value of getting these details right becomes more obvious. Each correct molecular formula, each confirmed weight, strengthens the foundation that new discoveries build upon.
Why Safety Goes Beyond the Label
People dealing with chemicals often expect clearly stated dangers on labels or in a safety data sheet. That expectation works for many well-studied compounds, but every now and then, a new or unusual chemical pops up—like 1-Carboxy-1-Methyl-Pyrrolidinium Bromide. This one doesn’t show up in many chemistry textbooks or safety manuals, so questions about its toxicity raise understandable concern.
What Does Science Say?
Right now, peer-reviewed science tells a patchy story. There’s only a sprinkling of toxicology data for this pyrrolidinium compound. What we do know comes from similar families of chemicals: pyrrolidinium salts and bromides. Some pyrrolidinium salts show low-to-moderate toxicity in rodents, depending on the substitutions attached to the ring. Bromide, on its own, is an old-school sedative that can lead to toxicity at high doses, especially if ingested for extended periods.
As for 1-Carboxy-1-Methyl-Pyrrolidinium Bromide itself, laboratory supply companies issue vague warnings referencing irritation risks upon contact with skin or eyes, and potential digestive distress if swallowed. That does line up with basic chemical hygiene principles. I’ve seen firsthand in research labs that assuming “safe enough” can backfire—especially with poorly studied substances.
Lab Risks and Real-World Exposure
Colleagues who have handled this sort of salt in organic synthesis wear full PPE, including gloves and goggles, and make sure they’re working in a fume hood. The dust from these salts can irritate the airways or skin, and powders in general should never be breathed in. It’s easy to be casual with bench chemistry, but a chemical like this deserves extra respect, especially without robust evidence. The same common sense goes for disposal; flushing unknowns down the drain can have ripple effects in the environment, given their unpredictable breakdown products.
Why Transparency Matters
Regulatory agencies like OSHA and the European Chemicals Agency push companies to test for acute toxicity, chronic exposure risks, and environmental fate before letting new stuff out into the world. In the case of 1-Carboxy-1-Methyl-Pyrrolidinium Bromide, it’s not on many major hazardous substances lists, which may give a false sense of security. Just because a substance isn't flagged by regulators doesn’t mean it’s harmless. Often, it only means no one has done the studies yet.
A lack of data forces chemists, manufacturers, and students to lean on precaution. The lessons I’ve learned over years in laboratories echo loudly here: test in small batches, ventilate well, document anything unusual, and treat every unknown with more caution than confidence.
Better Data, Better Decisions
Demand for safer chemicals keeps growing—especially as environmental and health watchdogs push for more information. Researchers need transparent reporting from chemical suppliers, plus peer-reviewed testing for acute and chronic toxicity and impact on water or soil. Training programs for workers must drive home the message: just because you’ve never seen a chemical on a list of toxins doesn’t mean it’s risk-free.
Risk may sound abstract until it becomes personal. Whether you’re at a bench or setting policy, the story of 1-Carboxy-1-Methyl-Pyrrolidinium Bromide drives home a point I’ve seen again and again: if you don’t know, don’t gamble.
Why Purity Matters
Quality always makes a difference, especially when it comes to the purity of a chemical or ingredient. High purity levels mean fewer surprises—no side reactions, no unwanted contaminants sneaking in from the manufacturing process. I have learned from experience that small impurities can cause big headaches. In pharmaceuticals, for example, less than a percent of impurity may turn a safe treatment into something useless at best or dangerous at worst. Back in my university lab days, I spent hours tracking down why an experiment failed, only to find a small impurity in a reagent caused the entire reaction to crash.
Quality control teams agree: The United States Pharmacopeia (USP) and similar organizations in other countries define acceptable purity standards for common ingredients. A product labeled "pharmaceutical grade" signals purity standards above 99%. If a buyer sees “technical grade” or “industrial grade,” they should expect more impurities and double-check what those might be. Some industries, such as electronics or food processing, demand these high grades to avoid product recalls or liability problems. Reports from the US Food and Drug Administration highlight cases where insufficient purity led to major recalls and cost companies millions—not to mention potential health risks down the line.
The Role of Form: Powder Versus Liquid
The decision between powder and liquid isn’t an afterthought. In my own work, I have found that powders travel better, store longer, and are often easier to measure out with accuracy. Powders can also be mixed into other materials or dissolved if needed. On the flip side, liquid forms offer fast absorption or easier application. If you’ve ever tried getting a fine powder to blend into water without clumps, you know it isn’t always a smooth experience. For instance, lab technicians often prefer liquids when precise dosing is necessary, since every milliliter delivers the same amount of active ingredient.
Both forms come with their challenges. Liquids can spoil if there’s a lot of water involved, and shipping costs increase when weight and volume go up. Powders, depending on how finely they are made, can make a mess or create dust that irritates lungs and skin. In my time handling chemicals in both forms, I always looked for sealed, labeled containers and checked the documentation for water content and carrier solvents. Some liquids contain stabilizers that keep things shelf-stable, but those extras can sometimes cause their own issues.
Practical Tips for Buyers
The safest move is to request the certificate of analysis from the supplier. This document spells out just how pure the product is, in percentage points, and lists harmful or even just annoying impurities. Good vendors make this document available up front, and reliable brands usually print the key purity data on their label for everyone to see. If you are using these materials for any regulated process—pharmaceuticals, health supplements, or food—it pays to stick to well-known suppliers and only order products with a full paper trail.
Anyone who’s ever dumped money into low-grade input material only to see production grind to a halt can tell you: purity and the right form save money, time, and frustration. Whether you’re mixing up a batch of medication, making soap on a small scale, or processing critical components for electronics, starting with the best material gives you a smoother run from start to finish.