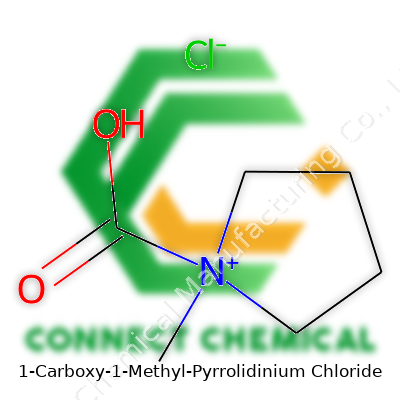
Understanding 1-Carboxy-1-Methyl-Pyrrolidinium Chloride
Historical Development
Research into pyrrolidinium salts has grown steadily since the late 20th century, driven by interest in ionic liquids and their promise across chemistry and green technology. Before this, focus tended to rest on older, more familiar ammonium salts and simple chlorides. Chemists began seeing the potential in complex nitrogen heterocycles like pyrrolidinium for their stability, tunable properties, and potential for new applications. Early syntheses were often labor-intensive, but refinements in organic synthesis and purification helped open doors. Over time, more academic labs published routes involving substituted pyrrolidiniums that paved the way for commercial manufacturers. Today, 1-Carboxy-1-Methyl-Pyrrolidinium Chloride springs from these decades of pushing boundaries in both basic and applied chemistry, driven by the need for materials with distinctive solubility and reactivity profiles.
Product Overview
1-Carboxy-1-Methyl-Pyrrolidinium Chloride falls under the group of quaternary ammonium salts, but its pyrrolidinium ring and carboxylate group lend a unique chemical fingerprint. It's sought in areas demanding solvents or electrolytes that handle extremes–whether that's water content, temperature, or aggressive chemical environments. This salt brings notable stability in water and many polar solvents. Its ionic nature supports use in electrochemical setups, analytical chemistry, as well as in organocatalysis and phase transfer catalysis. Some teams also use it as a structural building block for producing new functionalized pyrrolidinium compounds with targeted physical or biological traits.
Physical & Chemical Properties
This compound appears as a crystalline white solid that's readily soluble in water, forming a clear solution at room temperature. Its melting point often sits high enough to allow safe storage at ambient conditions, but avoids the difficulties associated with some hygroscopic chlorides. The molecule carries both a charged carboxylate and a quaternary ammonium center, making it zwitterionic in structure. That charge distribution gives it strong interactions with polar environments and disrupts crystallinity enough to enable its use in ionic liquids. Under mild heating it remains stable without risk of rapid decomposition, though long-term exposure to strong acids or bases may break it down.
Technical Specifications & Labeling
Manufacturers label this compound with identifying numbers such as CAS or EC, though purity, moisture content, and residual solvents draw much of the attention on certificates of analysis. Most commercial supplies target purity levels above 98%, and water content holds importance due to the compound’s high solubility. Labels frequently display hazard information consistent with GHS requirements, including any risks associated with eye or skin contact. Batch numbers and manufacture dates help trace products across the supply chain, which matters in regulated or sensitive applications where even minor impurities could skew results.
Preparation Method
Synthesis typically involves ring formation by reacting N-methylpyrrolidine with haloacetic acids under controlled temperatures, often in polar solvents. Some methods isolate intermediates like N-methylpyrrolidinium followed by carboxylation steps. After synthesis, the crude salt undergoes multiple washings and recrystallization from ethanol or water to remove unreacted starting materials and byproducts. The steps must control pH and temperature carefully, since the presence of chloride ions at high concentrations will impact the overall purity and yield of the desired salt. Many labs finish with filtration, drying under vacuum, and sometimes additional chromatographic purification to achieve the high standards for research or industry.
Chemical Reactions & Modifications
As a pyrrolidinium-based salt, this compound lends itself to a variety of functionalizations. The carboxylate group opens doors for substitution reactions, such as esterification or amidation, for further tailoring. Its quaternary ammonium functionality can be exchanged for other anions if different reactivity is needed, through metathesis with silver salts or ion exchange resins. It’s sufficiently robust for use in multi-step synthetic schemes, including those leading to ionic liquid crystals or as templates in the assembly of complex supramolecular architectures. Some labs focus on swapping the methyl group or introducing functional chains on the nitrogen, to optimize for solubility, melting point, or biological response.
Synonyms & Product Names
Chemical suppliers often list alternative names that refer to this molecule, such as 1-Methyl-1-Pyrrolidiniumcarboxylate Chloride, 1-Methyl-1-Carboxypyrrolidinium Chloride, or simply Pyrrolidiniumcarboxylic Acid Chloride. Trade labels may shorten or alter the sequence depending on naming conventions, though the underlying structure remains constant across reputable vendors. Knowing these synonyms helps researchers avoid misidentification and ensures cross-compatibility between labs and suppliers.
Safety & Operational Standards
Safety matters take front seat, and product literature lays out clear guidance. Material safety data sheets warn against prolonged contact with eyes and skin, and recommend gloves, goggles, and local ventilation. Spills are tackled with water or inert absorbent, with disposal following local regulations for quaternary ammonium salts. While not known to pose major acute hazards, the absence of long-term toxicology studies means anyone handling volumes over a few grams should show diligence. Container labeling, secondary containment, and standard chemical hygiene practices keep risks under control. Regular monitoring for residue or degradation ensures product quality stays high, especially in sensitive uses like pharmaceuticals or specialty synthesis.
Application Area
In my experience working with specialty chemicals, 1-Carboxy-1-Methyl-Pyrrolidinium Chloride stands out for performance as a phase-transfer catalyst and conductivity booster in electrochemical applications. Electroplating baths benefit from the salt’s stable, non-volatile nature, and researchers often employ it in testing ionic liquid mixtures where high polarity is critical. Analytical chemistry labs find value in its ability to stabilize charged intermediates during titrations. Pharmaceutical efforts sometimes tap it as a scaffold for drug modification, thanks to its dual ionic and hydrophilic character. Work in environmental remediation also explores these salts for capturing metal ions or organic toxins from water, leveraging the high charge density and water affinity.
Research & Development
Ongoing research digs deep into new uses, including solvent alternatives for green chemistry projects. Academic labs push boundaries on catalysis, using the salt’s unique ionic profile to speed reactions that other quaternary species struggle to support. Materials scientists run experiments on using pyrrolidinium salts in batteries and supercapacitors. Biochemists explore derivatized forms for selective binding of proteins or low-molecular-weight biomolecules. Consortia among academic groups and industry partners look for refinements in synthetic routes, aiming for fewer steps, higher yields, and less hazardous starting materials. Many in the field push for open data sharing on performance metrics and environmental impact so new applications rest on solid, transparent science.
Toxicity Research
Toxicology studies remain limited but offer early insights. Acute exposure through typical lab use shows low oral and dermal toxicity in animal models, but long-term exposure data lacks depth. Researchers tread carefully, especially where modified pyrrolidinium salts are involved, since some quaternary ammonium compounds cause irritation or bioaccumulation. Recent work focuses on degradation pathways in environmental settings, measuring persistence in surface water and evaluating breakdown products for any harmful behavior. Regulatory agencies and independent labs continue to seek a clearer picture on ecological impact, and safety sheets update as new results trickle in. Until more comprehensive data accumulates, the chemical community favors extra personal protection and lab ventilation, especially in educational settings or high-volume use environments.
Future Prospects
As the demand for advanced ionic liquids and green reagents accelerates, 1-Carboxy-1-Methyl-Pyrrolidinium Chloride looks set for greater relevance in next-generation batteries, removable catalysts, and precision medicine. My own time in collaborative projects suggests new electrolytes and novel solvents based on pyrrolidinium offer lower volatility and better tolerance of heat and water, vital for sustainable technology. Commercial scale-up still faces hurdles–including cost, waste minimization, and regulatory adaptation. Open-source protocols for synthesis and modification will help smaller labs and startups expand their reach, while joint research with toxicologists and environmental scientists promises a safer, clearer path for real-world rollout. Keeping focus on responsible sourcing, transparent reporting, and iterative product improvement, the field keeps moving toward a healthier relationship between innovation, sustainability, and global supply.
From the Lab Shelf to the Factory Floor
1-Carboxy-1-Methyl-Pyrrolidinium Chloride doesn’t exactly roll off the tongue. In the chemical world, though, it’s a name people remember for its reliable role in several tough processes. This salt packs a punch where scientists and engineers run up against stubborn obstacles, especially where regular organic compounds don’t quite pull their weight. For those who’ve spent hours sweating over experimental setups that refuse to budge, this chemical isn’t just a formula—it’s a sign of progress.
Improving Industrial Electrochemistry
One of the biggest draws comes in the field of electrochemistry. Researchers—myself included—have tinkered with a long list of electrolyte additives for batteries and fuel cells, aiming to squeeze out every bit of efficiency. 1-Carboxy-1-Methyl-Pyrrolidinium Chloride has come up often in studies focused on ionic conductivity and thermal stability. Its ionic liquid qualities help cut heat build-up and boost energy storage, leading to safer and longer-lasting lithium batteries. Given how power storage affects everything from smartphones to solar panels, an electrolyte that reduces the risk of meltdown or fire counts for a lot.
Helping Out in Organic Synthesis
Any chemist who’s tried to synthesize a tricky molecule knows the frustration of low yields and sluggish reactions. This compound, with its unique pyrrolidinium structure, can act as a phase-transfer catalyst. In plain language: it moves reactants into the best possible position so they can get to work quickly. Over time, I’ve found this approach can save hours—and expensive starting material. More and more industrial solvent systems switch to such salts, seeking both safety and better output. In an era where regulation and cost-cutting steer company priorities, chemicals that tick both boxes have real staying power.
Environmental and Green Chemistry Contributions
Sustainable processing gets harder each year. For every process that claims “green” status, there are five that still rely on solvents with questionable safety. 1-Carboxy-1-Methyl-Pyrrolidinium Chloride comes with low volatility and a well-documented toxicological profile. So, swapping in this compound replaces problematic solvents and reduces emissions, chipping away at contamination sources. Early in my career, efforts to move toward safer chemistry often felt symbolic—no one wanted to risk efficiency for an eco-friendly label. Now, industries find this switch cost-effective, and consumers look for products made with safer chemistry.
Potential Solutions and the Road Ahead
Challenges always show up. Sourcing pure material sometimes raises costs, especially in places with limited chemical infrastructure. Education falls short; many working chemists haven’t yet been trained to use newer ionic salts, leading to hesitation on the production floor. To close the loop, bringing more hands-on training and providing open access to updated toxicology and handling guides would help spread best practices. Companies can also invest in smarter synthesis—making this compound cheaply and at scale—so smaller manufacturers don’t get priced out. The more people work with chemicals like 1-Carboxy-1-Methyl-Pyrrolidinium Chloride, the faster safer, greener manufacturing takes root.
Where Applications Go Next
All these uses tie back to one point. Chemistry moves forward when tools do more than one job. After years of watching shifts in the lab and factory, I’ve seen how this one compound takes on multiple challenges: safer batteries, cleaner reactions, smaller environmental footprints. As technology keeps climbing, materials like this open new doors—and help close old ones that lead to avoidable risks and outdated thinking.
Beyond the Lab: What Sets This Molecule Apart
Ask most folks about 1-Carboxy-1-Methyl-Pyrrolidinium Chloride and you’ll get a blank stare. The name itself is a mouthful, and the chemical world doesn’t always do the best job making things approachable. Strip away the jargon and you see a molecule shaped by real forces—carbon, nitrogen, hydrogen, and chlorine—all pieced together in a ring that looks ordinary at first, but carries some clever tricks inside.
Breaking Down the Chemical Structure
The heart of this molecule is a five-membered ring called a pyrrolidinium. Imagine a pentagon of four carbon atoms, with a nitrogen atom closing the loop. On that ring, the nitrogen proudly holds a methyl group—a single straight-shot of carbon and hydrogen atoms. Right there on the same nitrogen, a carboxy group dangles, made of a carbon double-bonded to an oxygen and carrying an extra oxygen as an OH, bringing acidity to the party. Since the ring’s nitrogen winds up with more bonds than it should by standard chemistry rules, it carries a positive charge. Enter the chloride, sticking with the molecule as a counterweight, bringing balance so the whole mess holds together.
Why This Structure Matters
I first came across quaternary ammonium salts like these back in my university days. We were poking at new ionic liquids—a fancy term for salts that melt at low temperatures—and someone passed around a vial with a straw-colored liquid. It didn’t look like anything special, but our professor pointed out how these structures get used in everything from synthetic reactions to pharmaceuticals. The charged nitrogen alters how the molecule interacts with water, oils, or even the guts of a living cell. That flexibility means you get to tweak properties for all sorts of jobs.
These chemical quirks aren’t just fun facts. Teams working on greener solvents—liquids that clean or dissolve without trashing the environment—often turn to structures like this one. That carboxy group does double-duty: one side pulls water, making the salt dissolve well; the other lets chemists graft on new pieces or modify behavior. Across Europe and Asia, labs are spinning out new variations to clean up manufacturing, lower energy waste, and swap out old solvents that leave toxic residues.
Challenges on the Horizon
No chemical hits the shelves without a few bumps. Take the chloride part: in water, chloride doesn’t always play nice, especially at high concentrations. Factory runoff or lab waste can put stress on local ecosystems if not handled correctly. There’s also the simple cost of making the stuff. I spent a summer internship at a small specialty chemical maker, and we constantly weighed whether starting materials could be sourced affordably and whether by-products would cause headaches later. With pyrrolidinium salts, clean synthesis needs care, since leftover bits of base or acid push the purification costs up.
Possible Solutions Rooted in Real-World Chemistry
Anyone looking for progress here needs more than lab tricks. Smarter synthesis—using milder reagents and cleaner reactions—can cut cleanup costs and reduce waste. My mentors would often swap reaction steps, finding one-pot processes instead of multistep ones, slashing solvent use by half or more. Companies focused on recycling spent ionic liquids make a meaningful dent too, capturing and reusing precious chemicals before they touch groundwater or landfill.
For the long haul, education helps most. Young scientists won’t care about fancy molecule names if those molecules don’t connect to practical results. Seeing 1-Carboxy-1-Methyl-Pyrrolidinium Chloride not just as an abstract structure, but as a tool for cleaner manufacturing or safer pharmaceuticals, turns chemistry from a puzzle into a real-world pursuit. That’s where the next breakthrough usually begins.
Looking at the Substance
1-Carboxy-1-Methyl-Pyrrolidinium Chloride isn’t a familiar name for most people, but this chemical has quietly found a place in several industrial processes—catalysis and specialty synthesis top the list. If you've ever worked around research labs or custom manufacturing, you know how tough it can get to figure out which chemicals are genuinely dangerous and which just sound that way.
Having handled my share of unfamiliar bottles in labs, I approach every label with healthy skepticism. Search for peer-reviewed studies, skim through safety data sheets, and listen to chemical health experts long enough, and you notice that hazard is all about the details—solubility, stability, exposure, the whole package.
The Science and the Gaps
So, what does the science say? Sifting through public databases, I see a real lack of standardized toxicity data specific to 1-Carboxy-1-Methyl-Pyrrolidinium Chloride. That doesn’t mean the coast is clear, only that researchers haven’t given it the full toxicology workup. European Chemicals Agency and US EPA repositories don’t have strong hazard profiles for this chemical, probably because it’s used more in very niche applications, not in broad consumer markets.
Looking for clues, I checked the broader class—the pyrrolidinium salts group. Sometimes, these salts turn up as mild irritants for eyes or skin. But few published cases report anything close to acute toxicity for humans. Industrial users I’ve spoken with say the most common risk has more to do with lack of information or mishandling during reactions than any dramatic hazard.
Bigger Safety Picture
The biggest real-world issue? Unknowns. Every chemist I know will point out that the less you know about a compound, the more cautious you have to be. Short-term irritation can show up unexpectedly, especially if the dust or solution lands on bare skin. Without solid chronic exposure data, workers and lab techs stick to strict PPE protocols—gloves, goggles, fume hoods—regardless of how dangerous the label sounds. I remember a time a colleague decided to “skip” gloves with a new reagent judged as “low risk” on paper. A small spill turned into hours of irritation and a call to the on-site medical team. That shapes your approach fast.
Disposal brings another layer of questions. Without knowing the breakdown products in water or soil, most facilities run any waste through hazardous disposal channels. A study on similar salts noted they can react with common cleaning agents to form irritating fumes—another reason to take every new chemical seriously, even without obvious hazard labels.
Working Toward Answers
Most people want clear, actionable answers: is it risky or not? Without direct data, the precautionary principle wins out. Safety officers across the chemical industry call for more testing before giving compounds like 1-Carboxy-1-Methyl-Pyrrolidinium Chloride a clean bill of health. Until then, standardized data collection, transparent reporting, and open lines between those handling chemicals and those writing regulations make up our best protection.
If you’re dealing with new specialty chemicals in any setting, don’t take the unknowns lightly. Stick with strong procedures, and always fight for the data you need to keep people safe. An informed worker makes for a safer workplace, and in chemistry, a little extra caution always pays off.
Storing Chemicals Isn’t Just Following Rules—It’s About Safety and Shelf Life
People in the lab often treat storage as an afterthought. Toss a bottle on the right shelf, scribble the date, and hope for the best. Over years of working with specialty chemicals, I’ve seen how this attitude leads to ruined batches and, worse, unsafe environments. 1-Carboxy-1-Methyl-Pyrrolidinium Chloride deserves respect at every step, starting from storage. This is more than science—this is about protecting people and investments.
What Happens Outside the Bottle Matters
The recommended spot for 1-Carboxy-1-Methyl-Pyrrolidinium Chloride falls between cool, dry, and well-ventilated shelves. Labs that keep it away from direct sunlight, in tightly sealed containers, end up with fewer problems down the road. Moisture in the air can turn a sample lumpy or even trigger slow chemical changes that aren’t obvious at first glance. I’ve seen a few researchers ignore this until they notice something off in their results, and by that point, it’s too late to turn back time.
Room Temperature Isn’t a Guess
There’s a big difference between room temperature on paper and the back corner of a storage room in July. The sweet spot for 1-Carboxy-1-Methyl-Pyrrolidinium Chloride rests at about 20–25°C (68–77°F), but nobody benefits from storing it next to a heater or window. Real-world labs that use digital thermometers or, better yet, dedicated climate-controlled cabinets, see longer shelf life and fewer inconsistencies in experiments. Consistency comes from paying attention to these details.
Why Air and Humidity Wreck More Than Paper Labels
Leaving containers loosely closed invites moisture to join the party. I once worked with a supplier who didn’t tighten the cap on a shipment. The next week, the contents caked. Not only is caked powder hard to use, but it also suggests chemical breakdown. Desiccators change the game for these salts. Simple silica gel packs make a difference, and every extra seal helps.
Keep Oxidizers and Acids Away
Storing reactive chemicals together is a rookie mistake that even veterans sometimes make, especially under pressure. 1-Carboxy-1-Methyl-Pyrrolidinium Chloride needs its own space, separate from acids and oxidizing agents. I’ve seen enough to know unwanted reactions don’t just happen in beakers—they kick off in storage, sometimes quietly, with only a slight change in scent or color as a warning. A simple label and a dedicated spot save a lot of trouble.
Good Habits Save Money and Time
Frequent checks do more than satisfy paperwork requirements. Spotting a leaky cap, a cracked jar, or condensation inside the container saves time, money, and sometimes an entire project. Training everyone in the lab, not just the head chemist, means there is always an extra set of eyes catching small mistakes before they turn into expensive problems.
Building Better Practices
Respect for chemical storage often trickles down from the top. Supervisors who set clear protocols and invest in quality storage gear create labs where accidents stay rare. I’ve seen simple changes—like adding humidity monitors or regular cleaning schedules—turn a messy storeroom into a safe, reliable workspace. Such investments quickly pay off in less waste and fewer sick days.
Real safety and quality come down to these tried-and-true basics: seal it up, store it cool, keep it dry, and label it right. Those who treat every vial like it matters end up with the best results, time after time.
What Purity Means for a Chemical Like 1-Carboxy-1-Methyl-Pyrrolidinium Chloride
Chemical purity often looks like a dry topic, but it’s crucial for people in fields ranging from lab research to manufacturing. There’s a real difference between a compound taken off a shelf at “technical grade” and something you’d find at “analytical grade.” I’ve seen projects derailed by an unexpected contaminant or trace impurity. So, for a compound like 1-Carboxy-1-Methyl-Pyrrolidinium Chloride, purity isn’t just a marketing label—it can shape results, safety, and costs.
Availability: Does This Chemical Come in Multiple Purity Grades?
Companies that produce and supply 1-Carboxy-1-Methyl-Pyrrolidinium Chloride have learned that buyers’ needs don’t look the same. Sourcing from different vendors, researchers sometimes discover versions described as “laboratory grade,” “ACS grade,” or simply “purified.” One key reason for these distinctions comes down to the end use. Higher purity grades often serve labs where analytical accuracy matters most, such as pharmacology or battery development. Lower grades work fine for tasks like synthesis or initial product development, where small impurities won’t ruin the whole batch.
Why Purity Impacts Outcomes (and Budgets)
From my own experience, even small impurities can throw off a reaction. A single contaminant in a battery electrolyte will mess with charging cycles and safety profiles. At the same time, paying for “ultra pure” when the project allows a broader specification just eats the budget. Chemists juggling government grants do not shrug off a difference of several hundred dollars per kilogram.
According to widely published technical data, purity grades for chemicals often range from 95% (technical) to over 99.5% (high purity). For regulatory environments, such as pharmaceuticals or semiconductors, buyers ask for the highest grades with extensive testing and certification. I’ve seen TDS sheets from chemical suppliers spelling out every contaminant below 0.01% for customers that demand complete transparency.
Risks of the Wrong Purity
The flipside of multiple purity grades is confusion. Colleagues have shared stories of suppliers not listing impurities clearly, or customers mixing up product codes. One lab wasted weeks running diagnostics, only to trace their issue to a chloride contaminant not disclosed at ordering. With 1-Carboxy-1-Methyl-Pyrrolidinium Chloride being considered for advanced materials and energy storage research, hidden impurities quickly become a serious roadblock. Worse still, safety hazards rise when reactive contaminants sneak through—a small oversight can cause bigger headaches down the line.
Tools and Tips for Sourcing the Right Grade
Picking the right purity means reading past the glossy front page of a chemical catalogue. Ask suppliers for batch-specific certifications. Look at recent third-party analysis reports if they’re willing to share them. Forums, research papers, and fellow researchers can be surprising sources of leads when one’s supplier seems tight-lipped or unclear. Being precise with the intended use and testing needs helps cut through the generic sales talk and land on a grade that brings peace of mind to both researchers and managers signing the checks.
Solutions: How Researchers and Industry Get Purity Issues Under Control
Some labs now build standard operating procedures just for chemical vetting, demanding clear certificates and retaining samples for cross-testing. Industry leaders have begun standardizing product codes and quality control paperwork to help buyers quickly check what’s in stock. Leaning on international guidelines, such as those set by ISO or ASTM, brings consistency. Investing time up front saves headaches later, especially for breakthrough research or regulated manufacturing.