1-Carboxyethyl-3-Ethylimidazolium Bromide: Insight, Development, and Directions
Historical Development
Imidazolium-based ionic liquids started drawing interest from chemists and engineers in the late twentieth century, mainly because traditional solvents in many industries were causing both safety and environmental concerns. The search for alternatives led to a burst of research in the field of ionic liquids. With their low volatility and strong solvating power, these compounds began to fill a gap. The addition of functional side groups shaped both the versatility and specificity of these molecules; among them, 1-carboxyethyl-3-ethylimidazolium bromide stood out. Researchers collaborated across continents, sharing data and methodologies, with academic papers and patents rapidly increasing. This intersection of green chemistry, process efficiency, and new material design fueled ongoing projects in public and private labs alike. Many remember the first applications in catalytic systems and extraction processes, where this ionic liquid outperformed earlier mixtures. Interest did not wane—instead, it steadily built up behind improved synthesis, safety, and scale-up.
Product Overview
1-Carboxyethyl-3-ethylimidazolium bromide appears as a crystalline or viscous liquid, depending on hydration and purity. A simple vial of this compound packs unique features, rooted in the imidazolium core and the carboxyethyl side chain. This marriage shapes not only its solubility but also its ability to interact with a range of organic and inorganic species. The popularity among researchers owes much to its stability under normal laboratory conditions and its compatibility with a range of starting compounds. Suppliers ship this compound in sealed amber bottles to prevent degradation, with lot numbers and purity clearly indicated on labeling. Most scientists trust reliable vendors who offer robust quality documentation and traceable synthesis pathways—essential for everything from bench-top work to pilot-scale innovation.
Physical & Chemical Properties
At standard room temperature, 1-carboxyethyl-3-ethylimidazolium bromide often forms a white to off-white crystalline solid with a melting point hovering near 100°C, though the exact value shifts depending on residual water. It dissolves readily in water and polar organic solvents, reflecting its strong ionic character. Its density lands around 1.2–1.3 g/cm³, giving a reassuring heft in a standard laboratory vial. The bromide anion imparts certain reactivity benefits—helping with ion exchange and acting as a counter-ion in metathesis reactions. Electrochemical stability sits at the core of its usefulness, meaning it won’t break down easily during moderate reaction conditions. Infrared spectra reveal consistent marker peaks for the carboxyl and imidazolium functional groups, and nuclear magnetic resonance (NMR) data gives clear chemical shifts that confirm structure. Any manufacturer aiming for high quality focuses on moisture content and free bromide analysis, to minimize unwanted catalytic behavior in downstream processes.
Technical Specifications & Labeling
Every container of this ionic liquid includes batch tracking, purity details, and hazard identification numbers. Purity levels typically reach above 98%, verified by high-performance liquid chromatography and NMR. Safety sheets lay out hazard classes, storage recommendations, and emergency measures in clear language. Lot-specific analysis highlights residual water, heavy metals, and byproducts to reassure customers of precise composition. Labels display both systematic (IUPAC) and trade names, alongside recommended disposal protocols and shelf-life windows. Companies posting certificates of analysis from independent labs build the most trust—just like a good mechanic who shows you exactly what was fixed.
Preparation Method
Labs usually synthesize 1-carboxyethyl-3-ethylimidazolium bromide using a quaternization reaction. Start with 1-ethylimidazole and react it with 3-bromopropionic acid under mild conditions. The process often occurs in a water or acetonitrile solvent at temperatures just above room temperature. Reaction times average a few hours, with purification steps that include crystallization or solvent exchange. Using vacuum drying, chemists can remove residual solvents, minimizing impurities. Scale-up work has made the method both reliable and accessible, bringing the ionic liquid from bench to kilo-lab settings without major hiccups or safety issues. Researchers who work hands-on with these processes know that clean glassware, fresh reagents, and careful temperature control make the difference between high-quality product and troublesome side mixtures.
Chemical Reactions & Modifications
This ionic liquid opens doors due to both the imidazolium core and the functional carboxyethyl arm. In catalytic processes, the molecule can shuttle between actives sites, stabilizing transition states and helping with selectivity. Chemists have grafted the carboxyethyl group onto supports, anchoring the ionic liquid for reusability in flow reactors. In solvent extraction, the bromide can swap out for alternative anions, creating new ionic pairs with tailored properties. The molecule acts as more than just a solvent: it participates, coordinates, and sometimes functions as an organocatalyst. Reactions involving metal ions benefit from its unique charge balance, and modification by esters or amides further expands its reach. Cautious researchers catalog side reactions, but most report predictable outcomes, with straightforward workups.
Synonyms & Product Names
1-Carboxyethyl-3-ethylimidazolium bromide crops up in the literature under various names. Trade catalogs use descriptors like ‘[CEIM]Br’ or ‘Carboxyethyl-Et-Im-Br’, offering shorthand that saves time during searches. Some papers refer to it as ‘3-ethyl-1-(1-carboxyethyl)imidazolium bromide’, following IUPAC rules closely. Labs often pick one term, but researchers working across departments pick up on synonyms quickly. Unique product codes from manufacturers make ordering simple and traceability clear, especially on repeat orders where quick turnaround matters.
Safety & Operational Standards
Handling any ionic liquid calls for attention, and 1-carboxyethyl-3-ethylimidazolium bromide is no exception. Operators wear gloves, splash goggles, and lab coats, and work in vented hoods to minimize eye or skin contact. Store the containers tightly closed, away from heat or acidic vapors, as the bromide ion can slowly corrode metal surfaces. Material safety data sheets highlight low flammability but ask users to keep sources of ignition far away. In the event of a spill, absorbent materials and water dilution help, followed by disposal as hazardous chemical waste. My own experience in the lab underscores the importance of clear labeling and emergency wash stations—just a simple error, like working without gloves, can take days to recover. Most university and industrial labs run annual training to stay ahead of any issues, emphasizing real examples rather than just paper protocols. Open reporting of near-miss incidents builds a stronger safety culture and prevents small errors from turning into serious injuries.
Application Area
1-Carboxyethyl-3-ethylimidazolium bromide works across chemical synthesis, separations, and catalysis. In academic labs, this compound streamlines reaction setups, offering tunable solvation for organic and organometallic reactions. In pharmaceutical projects, the presence of ionic liquids has trimmed waste and simplified extraction of target molecules. Companies in environmental science turn to these chemicals for removing pollutants in water treatment. Recent patents describe uses in bio-catalytic processes and electric battery research. Lecturers teaching green chemistry often demonstrate how the right ionic liquid shrinks a multiple-step process to a handful of simple actions. I have seen industry collaborators applying it to capture carbon dioxide, indicating new hope for practical solutions to big problems. The range of uses seems only limited by imagination and willingness to test new approaches. Tech transfer from bench to factory floor still takes hard work, and cost remains a factor, but the evidence points to steadily increasing adoption.
Research & Development
Teams focusing on improving ionic liquid properties regularly design analogues by tweaking the cation or swapping out the anion. Academic partnerships often lead efforts, with Ph.D. students tackling the fine-tuning of synthesis and characterization. I have seen countless presentations where researchers display thermal stabilities, viscosity data, and phase diagrams, using this compound as a benchmark. Big research projects target corrosion inhibition, selective extraction, or biomolecule stabilization, relying on the predictable performance of 1-carboxyethyl-3-ethylimidazolium bromide. Collaborations with industry push for lower-cost production and more sustainable feedstocks, echoing trends toward circular economy principles. The compound finds itself as a reference standard in comparative studies, showcasing advantages and limitations side-by-side with competitors. My contacts in Europe mention ongoing regulatory reviews, driven both by success stories and concerns about lifecycle impacts.
Toxicity Research
Preliminary studies on toxicity point to moderate biological effects, especially in aquatic environments. Researchers regularly publish bioassays on water fleas, fish embryos, and soil microbes, watching for both acute and chronic impacts. The carboxyethyl side chain often shows lower toxicity than longer-chain analogues, making it a candidate for greener chemistry projects. Analytical chemists track metabolites and breakdown products, trying to map environmental fate. In my work, I found well-managed disposal routes play a key role in preventing unwanted consequences; an accidental drain of even small quantities can stress local wastewater systems. Regulatory agencies demand clearer data on cumulative effects, spurring long-term studies both inside and outside of academic labs. No chemical tool is risk-free, but the robust database continues to grow, driving better use and sharper handling guidelines.
Future Prospects
Looking ahead, this compound will probably help unlock more efficient chemical processes and spur innovation in materials science. Its blend of functional versatility and manageable safety profile makes it a logical choice for new applications. Most of my colleagues see strong opportunities in battery electrolytes, supported by steady gains in purity and processability. Computational chemists model new derivatives on supercomputers, searching for even lower toxicity and higher performance. Suppliers ramp up efforts on greener synthesis routes, aiming to replace hazardous precursors with renewable feedstocks. Tech licensing deals will likely expand, particularly as scale-up challenges fall away. I have seen these breakthroughs spark new conversations across academic, regulatory, and commercial circles—the kind of team effort where science and application meet. Trade groups pay attention, updating best practice manuals and sponsoring symposiums to share findings. The march toward safer, cleaner, and more productive chemical tools continues, with 1-carboxyethyl-3-ethylimidazolium bromide sitting firmly in the mix.
Chemical Synthesis on a Cleaner Path
Years of tinkering in the lab have taught me that efficient syntheses matter, but safer pathways matter more. 1-Carboxyethyl-3-ethylimidazolium bromide, once a mouthful you learn to pronounce, keeps finding its place on the benches of researchers pushing for greener chemistry. It shows up as an ionic liquid—basically, a salt that stays liquid at room temperature. This matters because old-school solvents like benzene or chloroform do real harm to folks and the environment. Take Suzuki or Heck reactions, for example: rather than reaching for flammable organic solvents, experienced chemists pick this ionic liquid to dissolve reactants, drive reactions, and cut down on hazardous byproducts. It delivers good yields, and you can recover and reuse it, so you save resources and keep the waste drum lighter.
Boosting Catalysis in Industrial Processes
Big plants need catalysts that keep reactions running at top speed without eating up more resources than they save. 1-Carboxyethyl-3-ethylimidazolium bromide helps dissolve both organic and inorganic ingredients in stubborn mixtures. In my time working on process development, using this ionic liquid in pilot trials improved reaction rates and made tough separations easier. Think of it as a tool that helps stubborn molecules mingle, so the valuable stuff comes out faster and purer. The bonus: it won’t evaporate into the air or explode when the temperature climbs, increasing safety for workers.
Taming Biomass and Extracting Value
One of the big challenges in turning biomass into something useful is breaking apart tough natural materials like cellulose. Ionic liquids step in here again. Folks working in biofuels, who sweat over the numbers on energy in and out, have discovered 1-carboxyethyl-3-ethylimidazolium bromide chops tough plant fibers into sugars better than plenty of other solvents. The research at the university I collaborated with leveraged this ionic liquid to extract pure cellulose from wood pulp. They could skip harsh acids or bases, keep gear from corroding, and cut the energy bill.
Separation and Purification without the Hassle
Clean separation saves time and cash in everything from pharmaceuticals to electronics. Processes that used to guzzle gallons of toxic solvents now switch to 1-carboxyethyl-3-ethylimidazolium bromide to do the work more safely. For example, separating metals like copper or nickel from mine waste becomes cleaner and more efficient. In one project, using this ionic liquid helped pull out rare earth elements from electronic scrap. Recovery rates went up, and we didn’t need to equip the building with extra ventilation, since this liquid doesn’t fume up the place.
Looking Forward: Safer, Smarter Chemistry
From what I’ve seen, this ionic liquid keeps gathering fans because it works hard on two fronts: delivering strong results and making labs more sustainable. The price can still pinch, especially in large-scale use, but ongoing research aims to lower costs and inspect long-term safety beyond the bench. If industries keep pushing for nontoxic, reusable solvents, you’ll see even more reasons to reach for 1-carboxyethyl-3-ethylimidazolium bromide, whether you’re making next-generation batteries, breaking down plants, or cleaning up old waste streams.
Understanding the Heart of the Molecule
Watching the chemical world evolve each day, the emergence of new ionic liquids like 1-carboxyethyl-3-ethylimidazolium bromide stands out for several reasons. This compound is an ionic liquid, which means it often stays liquid at room temperature because of its unique structure built from organic and inorganic ions. I remember my first encounter with ionic liquids in a university lab—surprised by how their salt-like roots could behave nothing like table salt, but much closer to honey or water depending on their makeup.
Chemical Structure in Simpler Terms
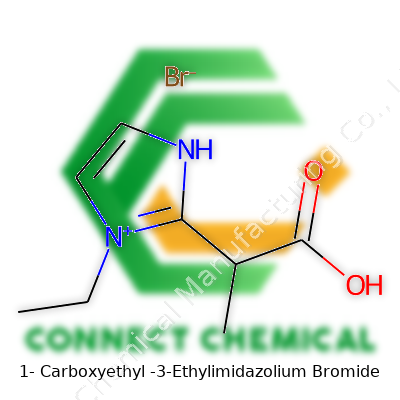
Rather than hiding behind a wall of technical jargon, I'll lay out what makes this molecule special. At its core sits the imidazolium ring—a five-membered ring with two nitrogen atoms sitting in the mix. On this ring, an ethyl group (–C2H5) attaches at one nitrogen, and a 1-carboxyethyl group (essentially a lactic acid fragment, –CH(CH3)COOH) sticks onto the adjacent carbon.
On top of that, bromide (Br⁻) balances the positive charge formed in the imidazolium structure, making the compound stable in solid and liquid forms. Visualizing this molecule is much easier once you remember the imidazole ring as the backbone, with its different side chains changing the personality and reactivity of the molecule.
Molecular Formula and Weight
Digging into the numbers, the molecular formula comes to C9H15N2O2Br. The molecular weight, measured in grams per mole, lands around 279.13 g/mol.
Here's how it breaks down:
- Carbon (C): 9 atoms × 12.01 = 108.09 g/mol
- Hydrogen (H): 15 atoms × 1.01 = 15.15 g/mol
- Nitrogen (N): 2 atoms × 14.01 = 28.02 g/mol
- Oxygen (O): 2 atoms × 16.00 = 32.00 g/mol
- Bromine (Br): 1 atom × 79.90 = 79.90 g/mol
Add those up, and you reach the noted value. This figure serves chemists, researchers, and manufacturers who rely on precise calculations for reaction setup and purity analysis.
Why Understanding Structure and Weight Matters
Ionic liquids such as this one often show up in discussions about green chemistry. Because of their ionic nature, they can act as versatile solvents—sometimes replacing more hazardous organic solvents in chemical synthesis or electrochemistry. I’ve seen these adopted in research labs aiming for cleaner reaction times and higher product recovery, not to mention their low vapor pressure means less pollution drifts into the air.
The carboxyethyl group tacked onto this imidazolium ring enables unique hydrogen-bonding interactions, which can change the way chemical reactions progress. A minor tweak in structure brings a huge impact—changing solubility, thermal stability, and the ability to separate target products.
Challenges and Moving Forward
Despite the appeal, large-scale adoption of ionic liquids tends to get bogged down by their sometimes stubborn synthesis routes and the need to handle halide ions responsibly. Bromide, while useful, can create regulatory headaches and waste-disposal costs. Synthetic chemists—myself included—often weigh environmental and economic concerns, seeking to swap bromide for more benign counterions or streamline purification.
There’s interest in building task-specific ionic liquids by modifying the basic imidazolium ring, making them better suited for particular uses from battery technology to pharmaceuticals. Setting up collaborations between academic research and industrial-scale producers could make cleaner, safer ionic liquids like 1-carboxyethyl-3-ethylimidazolium bromide a common tool.
Understanding the Substance Up Close
Facing unfamiliar chemicals in the lab always gets my attention, and 1-Carboxyethyl-3-ethylimidazolium bromide stands out. This ionic liquid fits into a growing class of salts, often praised for low volatility and flexibility in applications—from solvents to catalysis. Its usefulness draws in plenty of researchers and industry folks, but safety still calls for respect, not shortcuts.
How Exposure Hits Home
I learned quickly early in my research career that chemical exposure can creep up in small moments. Even though 1-Carboxyethyl-3-ethylimidazolium bromide doesn’t boil or evaporate the way volatile organic solvents do, that doesn’t mean danger disappears. A splash on bare skin, inhalation of fine powder during weighing, or even a poorly labeled bottle on a cluttered bench—these are where incidents usually start.
Acute toxicity studies on imidazolium-based ionic liquids show they can irritate the eyes, skin, and respiratory tract. A 2014 study in Chemosphere found that some imidazolium salts cause moderate toxicity to aquatic organisms and skin cells. Even though the carboxyethyl group adds some hydrophilicity, bromides can still act as irritants or cause more serious issues if mishandled. Long-term effects are less understood; that alone should encourage a careful approach.
No Substitute for Good Practice
In my own routines, safe handling of ionic liquids starts with the basics: gloves, safety goggles, and a sturdy lab coat. I always make sure to work in a well-ventilated space or fume hood, whether mixing up 10 grams or 100. Accidents rarely announce themselves, and cleanup takes less time than a trip to the emergency room.
Some ionic liquids stain skin for days. Others cause redness or cracked fingertips. I once wiped up a small spill with a gloveless hand, thinking “it’s just salt”—wrong move. Skin felt raw for hours. Since then, I double-check that gloves fit tight and clean up all residues, even those paper towels I used for cleaning.
Labeling and Waste Management Matter
Containers should always sport clear labels, including both chemical name and a summary of risks. I’ve seen accidents start from “mystery bottles” and faded tags. Disposal never goes down the sink or in regular trash. Most local regulations treat imidazolium bromides as hazardous: sealed in designated waste containers, and sent for professional disposal. Such habits keep the lab safe and the downstream environment healthier.
Solutions That Build Trust and Confidence
Access to clear safety data sheets (SDS) changes everything. Before using a new chemical, I dig into its SDS for instructions on spills, fire, and first-aid. Labs benefit when these are posted right where people work, not buried “somewhere in the files.” Team training also pays off. I’ve seen strong safety cultures start from informal walkthroughs, not just paperwork drills.
Chemists and industry users can boost safety by sharing incident stories and near-misses. Investing time in risk assessment pays huge dividends. Protective gear, up-to-date signage, careful disposal, and quick cleanup—these keep hazards in check. 1-Carboxyethyl-3-ethylimidazolium bromide fits into the broader picture of chemical safety. Tools might evolve, but discipline and shared habits keep the people behind the progress safe and healthy.
Why Storage Matters for Ionic Liquids
There’s no denying the challenges of working with modern ionic liquids, especially ones like 1-Carboxyethyl-3-Ethylimidazolium Bromide. The stability of this compound can go south fast if folks overlook a few key storage rules. I’ve seen well-meaning labs push their luck, only to crack open a bottle weeks later and find something off—clumps, discoloration, or a smell that should not belong. It’s not just about ticking boxes for compliance; it’s about respecting the chemistry in front of us.
Light Isn’t Your Friend
Direct sunlight acts like a slow-burn troublemaker for so many chemical compounds. 1-Carboxyethyl-3-Ethylimidazolium Bromide is no different. Light can trigger reactions slowly, degrading the structure and damaging purity. I remember a colleague hoping a dark brown bottle would do the job, but storage near a sunlit window still let enough light leak through to start trouble. For my own work, I stick with an amber-glass container and keep it tucked into a closed cabinet. Blocking those UV rays is one of the easiest ways to hold on to chemical integrity.
Keep It Cool, Not Cold
Heat picks apart ionic liquids in ways that really hurt long-term performance. But chilling them too much has its drawbacks; water can condense in cold environments, and that’s a ticket to hydrolysis and contamination. Through some testing, the sweet spot lands at room temperature—ideally between 18 and 25 degrees Celsius. Avoiding heat spikes from radiators or direct sources is key. I once lost a whole batch by storing it in a supply closet right above a server rack. Lesson learned: Check where heat sources are hiding in the lab or warehouse.
Tight Seals Beat Humidity
Open air is the silent enemy here. Ionic liquids soak up moisture from the atmosphere, almost like a sponge. With enough humidity, purity falls fast and hydrolysis turns into a genuine risk. It becomes even worse during wet months or in coastal labs. Using a screw-cap vial with a PTFE liner has made a world of difference for me. The liner blocks out water vapor and the tight closure limits other contaminants. I also add silica gel packets inside the storage container—an old trick, but it pays off every time.
Labeling Isn’t Optional
I used to think I would always remember what was inside each bottle, until the day I grabbed the wrong one. Clear, bold labels with full chemical names, dates received, and expiration predictions eliminate guesswork later. Add a note on handling precautions for the next user, especially if there’s a turnover in staff or shared chemistry space.
Room for Improvement
Not every lab invests in climate-controlled storage, especially if budgets are tight. Some workarounds can help—insulated cabinets, regular inventory checks, or pooling resources for a shared storage unit among nearby labs. Larger facilities sometimes offer loaner space in their temperature-monitored rooms, if you’re willing to ask.
Respect for the Material
In my own experience, the recipes for avoiding disaster haven’t changed much over the years: control the light, monitor the temperature, guard against water, and document everything. Mishaps cost more than just the lost reagent—they waste time and can put big projects on ice. Setting up a reliable storage approach up front means you spend less time troubleshooting surprises later.
Understanding Purity in Chemical Supply
Chemists tackling complex syntheses or building ionic liquids want one thing from their suppliers: confidence in purity. No one enjoys opening a bottle of 1-Carboxyethyl-3-Ethylimidazolium Bromide and discovering impurity peaks on a spectra—or worse—stalled reactions. On this front, suppliers usually offer purity levels above 97%. Solid NMR and HPLC data back those claims, so researchers aren’t left wondering if anomalies in their experiments are due to sloppy starting materials.
I’ve run experiments where even a 2% impurity led to whole days wasted. Trying to troubleshoot that mess rewrites deadlines and burns through grant budgets fast. In my experience, consistently high-purity chemicals are non-negotiable. A strong supplier provides data sheets on each batch, so customers see the numbers, not just marketing fluff. That transparency keeps research moving and supports reproducibility, especially when results get published or products reach the next stage.
Packing Up Choices: How Suppliers Approach Sizing
Choice of packaging size may look dull at first glance, but it shapes budgets, storage practices, and waste output. From my own lab years, nobody wanted to pay for a kilogram when all we needed was a few grams. Labs working on method optimization or screening experiments rely on small vials—5 grams or 10 grams per bottle make perfect sense. For process chemistry, the demand ramps up, so 25-gram and 100-gram containers become practical. Scaled-up industry applications sometimes go straight to bulk drums, but most suppliers keep their shelf bottles between 5 grams and 500 grams.
There’s another layer to packaging—integrity and safety. Moisture in the bottle spells disaster for ionic liquids and melts, turning quality product into an expensive mess. Reliable packaging means airtight seals and protective liners, especially when the product attracts water or reacts with light. Good suppliers pack chemicals under dry nitrogen and store them in amber glass. This isn’t just best practice—it prevents breakdown and unexpected color changes sitting on the shelf.
Why All of This Matters
Lab results follow a domino effect. One compromised reagent pulls down every step of an experiment. For companies scaling up, a single contaminated batch might run up losses in the thousands. That’s why buyers check two items on every chemical order: how pure is it, and how is it packed?
Regular audits, validated test results, and batch-specific certificates of analysis all contribute to trust. Over the years, I learned to avoid suppliers who ducked direct questions about their specs. Open communication alongside hard data makes the difference between wasted time and breakthrough results. In this field, cutting corners on raw materials doesn’t pay off. So clear labeling, strict QC, and reasonable sizes help researchers and manufacturers keep plans on track, avoid unnecessary waste, and push science forward without surprises.
Moving Toward Better Practice
Quality starts with the vendor but ends in the lab. Even small teams can negotiate for custom fill-size if their needs fall outside the catalog. For research groups, putting pressure on suppliers to share lot-specific purity checks means more reliable science. Industry groups and academic consortia are pushing hard for increased transparency along the supply chain—detailed certificates, full traceability, and better hazard communication. And it’s working: suppliers who meet these expectations gain more repeat business and stronger reputations. Basically, higher standards benefit everyone, from bench scientist to global manufacturer.