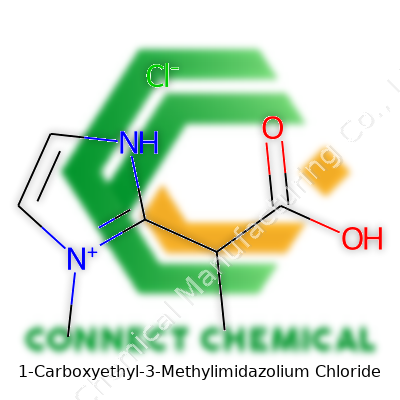
Drilling Down into 1-Carboxyethyl-3-Methylimidazolium Chloride: A Living Chemical Story
Historical Development
Back in the late 1990s, chemists all over the globe started searching for compounds that could carry the hope for safer, cleaner chemistry. Traditional solvents and catalysts, often volatile and polluting, left everyone breathing fumes and worrying about contamination. As scientists looked for answers, ionic liquids caught their eye—especially those based on imidazolium. 1-Carboxyethyl-3-methylimidazolium chloride didn’t show up by accident. It grew out of a broader push from researchers who built off early ionic liquid breakthroughs by Wilkes and Zaworotko, and then kept tinkering with the imidazolium core. Over time, this specific salt, with its unique structure, showed potential for both safer lab practices and greener industrial work. Things moved fast as benchmark papers and patents built momentum, pushing this compound from curiosity to contender in less than a decade.
Product Overview
1-Carboxyethyl-3-methylimidazolium chloride stands out among ionic liquids due to its chemical stability and practical versatility. You spot its white to off-white, hygroscopic crystalline form in labs where folks are edgy about handling anything volatile. The product brings a distinct edge with its solubility in water and several organic solvents, which means researchers can bring down the number of different chemicals they need to stock. Most suppliers package it in super-sealed, moisture-proof containers, with batch codes for tracking, and detailed certificates of analysis. The unique ionic pairing gives it a profile that fits the demands of both large-scale syntheses and detailed, bench-scale experimentation.
Physical & Chemical Properties
This compound holds appeal due to a melting range that stays comfortably above freezing but below the temperatures where organics break down. It doesn’t fume, it doesn’t have the punchy aroma typical of chlorinated solvents, and it mixes smoothly with water, alcohols, and polar aprotic solvents. The chloride ion helps with conductive properties, which opens doors in fields like electrochemistry. Density and refractive index hover at points convenient for quick lab work, and thermal stability helps prevent accidents under modest heating. Non-flammability reduces headaches with fire regulations, and because the carboxyethyl group blocks the formation of toxic byproducts, handlers have more confidence—less chance of something unpredictable in the fume hood.
Technical Specifications & Labeling
Manufacturers commonly assign batch numbers, purity grades (like 98% and above), and water content figures straight on their certificates. One often sees storage requirements spelled out—keep dry, shielded from sunlight, and preferably at room temperature. Labels flag the GHS (Globally Harmonized System) warnings, even if hazard levels fall into lower bands. QR codes bring up lot-specific spectral data. The CAS number helps avoid mislabeling, which matters in busy labs where similar-sounding chemicals sit on the same shelf. These steps anchor product trust among both academic and industrial customers.
Preparation Method
Most preparation paths start with 1-methylimidazole and react it with a pre-synthesized 2-chloropropionic acid (or similarly functionalized precursors). This process leans on direct alkylation under controlled temperature and pressure, often with careful base control to reduce impurity formation. Procedural refinements, including solvent-free paths, cut down on waste. Filtration and crystallization follow to separate the main product from side products and excess reagents. Final drying under vacuum ensures the lowest possible moisture content, which holds importance because residual water can alter the ionic liquid’s behavior in experiments or synthesis work.
Chemical Reactions & Modifications
One thing chemists seem to enjoy about this compound is its flexibility as both a reactant and a medium. The carboxy group acts as a functional point for esterification, amidation, and salt formation. The chloride anion can take part in metathesis or exchange reactions, especially where another anion suits a different purpose. Some groups have tinkered with the imidazolium ring itself, swapping methyls or chain-extending to give related derivatives. This versatility feeds back into everyday work in catalysis, electroanalysis, and bioconjugation. You also see the compound leveraged for stabilizing enzymes, scavenging specific metals, or anchoring new ligands for specialized tasks.
Synonyms & Product Names
Getting lost in a jungle of chemical names isn’t new, but this one carries aliases like 1-(1-carboxyethyl)-3-methylimidazolium chloride, CEMIM-Cl, and 3-methyl-1-(1-carboxyethyl)imidazolium chloride. Some catalogs abbreviate to CMI-Cl or use their in-house brand, but the core structure always runs back to the same imidazolium head with its sidechain twist. Looking up the standard CAS number remains the best way to cross-check what you’ve ordered, especially if you veer across European, North American, or Asian suppliers.
Safety & Operational Standards
From a safety point of view, one of the solid features of this compound is its low volatility and mild toxicity profile compared with many traditional aprotic solvents. Precaution never hurts, so glove use, splash-resistant goggles, and working in ventilated areas stay the norm, reducing the already minimal risk of skin or eye irritation. Detailed SDS files from sellers cover acute and chronic effects, disposal routes, and accident responses—no one should skip this deskwork. Facilities dedicated to ionic liquid use usually set up specialized waste streams, since the chloride component and residual imidazolium fractions warrant careful disposal. Engineering controls like containment pans or vapor traps come in handy in case a batch spills or decomposes unexpectedly.
Application Area
If you spend time in research labs or industrial pilot plants, you spot this compound popping up anywhere ionic character or non-volatile conditions matter. Synthetic organic chemists like it for running reactions that don’t tolerate water or oxygen, giving better yields and cleaner purifications. Electrochemistry sees plenty of use—the ionic conductivity and chemical inertness match up well with electrode processes. Some teams use it to dissolve and handle cellulose, getting around nasty acids or extreme bases. Others throw it into enzymatic work, allowing sensitive proteins to hold their structure or interact with new substrates. In separation science, the unique polarity smooths out problem extractions or cuts down on solvent use. Drug formulation groups have also started eyeing it as a solubilizing matrix, testing if it can boost poorly soluble actives in advanced delivery systems.
Research & Development
The constant curiosity in academic and industrial settings keeps this molecule under the spotlight. Every year, journal indexes fill up with new tweaks and uses—solid-phase reactions, energy storage, green catalysis, everything from material science to pharmaceutical synthesis. I’ve watched teams make big leaps by switching from standard organic solvents to this ionic liquid, seeing both improved safety for handlers and better performance for new reactions. Funding agencies push projects building better ionic liquids from this backbone, so the pipeline for new hybrids and applications keeps growing. Professional conferences now regularly feature talks on 1-carboxyethyl-3-methylimidazolium chloride as a reference compound and a launchpad for greener chemical technologies.
Toxicity Research
One thing that’s stood out over recent years is the attention paid to toxicity and ecological impacts. While ionic liquids, as a class, run with better human safety than chlorinated organic solvents, their effect on aquatic environments and soil systems draws research interest. Chronic exposure tests for this compound show low bioaccumulation and relatively quick breakdown under typical remediation conditions. In-vitro and in-vivo studies report moderate acute toxicity if ingested or inhaled in significant doses, but otherwise no severe mutagenic or carcinogenic trends. Research pushes for even safer analogs, drawing lessons from early findings—lowering ionic radii, testing renewable feedstocks, and designing break-down pathways that make future disposal more responsible.
Future Prospects
Looking forward, 1-carboxyethyl-3-methylimidazolium chloride isn’t just a chemical—it's a real-world answer to cleaner, safer, and more creative chemistry. Everyone from government agencies to graduate students wants compounds like this to change how synthesis, manufacturing, and environmental science get done. Large companies eye new uses in plastics recycling, electrolytic devices, and even as alternatives for toxic solvents in electronics manufacturing. Academic sources predict more derivatives and broader regulatory acceptance, given the compound’s favorable reputation. If trends hold, we’ll probably see this class of ionic liquids growing in areas from carbon capture to precision medicine. Real progress depends on not just keeping up with regulations, but pushing past them to genuinely safer, smarter chemical systems—this compound has already proven its value as a building block for that kind of future.
Unlocking Cleaner Chemistry in the Lab
Back in college, someone once told me lab work is half patience and half using the right compounds. 1-Carboxyethyl-3-Methylimidazolium Chloride—quite a mouthful—doesn’t just collect dust on shelves. Chemists, researchers, and engineers make the most of it when they want efficient, eco-friendly solvent action. Traditional solvents often mean putting up with volatile organic compounds, the sort you need a fume hood for. This compound brings a welcome change: less stink, less worry about inhalation, and a nod to the green chemistry movement. Researchers at green chemistry conferences have pushed for swapping harmful solvents for ionic liquids like this one, since they cut down on emissions and fire risk at the same time.
Energy Storage and Battery Research
If you’re passionate about clean energy, solid-state batteries and new kinds of supercapacitors always grab your attention. The electricity storage field keeps chasing after materials with low volatility and high thermal stability. Here, 1-Carboxyethyl-3-Methylimidazolium Chloride steps in as a solid electrolyte component. Everyday devices—phones, electric cars, maybe even your electric bike—get safer when researchers use less flammable ionic liquids in their batteries. Industry-backed studies in China and Germany echo a clear trend: this compound’s ionic conductivity compares well against older salts, all while keeping electrical systems a lot safer at high temperatures.
Cleaner Processing in Pharmaceutical Manufacturing
There’s plenty of talk about the high cost and slow pace of drug development. Take any pharmaceutical plant tour and you’ll see operations designed to reduce waste and save money. Switching over to ionic liquids for certain synthesis steps helps. 1-Carboxyethyl-3-Methylimidazolium Chloride acts as both solvent and catalyst in some reactions. In real terms, this means lower production of hazardous byproducts. European pharma companies reported up to 40% less solvent waste after moving to these ionic liquids for particular purification tasks. That’s a win not just for the environment, but also for any business counting operating costs.
Biomass Conversion and Biofuel Production
Sitting through a panel at an energy conference a few years back, I learned how hard it is to turn plant matter into fuel. Biomass doesn’t give up its sugars easily, which slows down any plan for making bioethanol. Here, specialty ionic liquids help break down stubborn cellulose into usable sugars. 1-Carboxyethyl-3-Methylimidazolium Chloride features in several case studies as a pretreatment solvent for converting straw, corn cobs, or bagasse into fuel. Researchers in Brazil and the US have shared data on higher conversion yields and simplified downstream processing after using this compound. For rural communities or countries leaning into biofuels, better yields mean more affordable, local fuel options.
What Could Improve its Impact?
If there’s one lesson I’ve picked up from green tech conferences, it’s that progress needs teamwork. The biggest barrier for 1-Carboxyethyl-3-Methylimidazolium Chloride involves price and large-scale supply. Manufacturers need processes that use less energy and create fewer leftovers. Policies that encourage industry labs and universities to share data push the field forward. More research into recyclability and biodegradation would also shrink environmental concerns. Technology keeps moving, and smarter use of compounds like this one will keep labs safer and manufacturing a little cleaner.
Understanding Purity in Chemical Supply
Purity always stands out in chemical products because impurities risk derailing years of effort. Nobody who spends their days in the lab wants to realize a trace contaminant fouled a reaction. Anyone sourcing 1-Carboxyethyl-3-methylimidazolium chloride should check for purity levels above 97%. Anything less often hints at sloppy handling or a supplier unwilling to do the hard work needed to ensure a clean compound. Purity isn’t a mere number; each percent closer to 100 means less chance of unwanted reactivity or skewed results.
Why Purity Directly Impacts Your Results
Every researcher I know checks the fine print when ordering ionic liquids. Impurities hide as tiny byproducts, solvents left behind, or leftover starting materials. For ionic liquids, extra water or chloride salts tip the balance of solubility, thermal stability, and catalytic properties. If you’re developing a new electrolyte, or testing out some catalysis, low purity could throw off data or worse, force a whole round of troubleshooting nobody budgeted for.
A supplier with serious credentials sticks to high-performance liquid chromatography (HPLC) or NMR checks to confirm what’s in the bottle. If I see a certificate of analysis and regular batch testing, trust goes up. Some companies openly share their testing methods and how they control contamination, even inviting outside audits. Those are the sort of partners that make researchers and product developers rest easier.
E-E-A-T Principles Put to Work
Many people overlook how Google’s E-E-A-T principles — expertise, experience, authority, trustworthiness — slip into purchasing lab chemicals. Anyone with a couple of years working hands-on can sense the difference between spec sheets full of fluff and those ready to answer the hard questions. Trust grows from suppliers who are transparent about their product’s origins, methods, and most importantly, real-world performance data — not just lab-bench theory.
If a business posts actual results from peer-reviewed studies using their 1-carboxyethyl-3-methylimidazolium chloride, or references customer feedback where purity solved an otherwise sticky problem, confidence follows. Purity claims backed by spectroscopy reports and direct customer stories do more than words like “high-quality” ever could.
Fixing the Purity Problem
The best fix comes from asking for and reading batch certificates, not just trusting catalog numbers. I once hit a roadblock after a routine NMR showed mystery peaks in what was supposed to be >98% pure salt. Calling the supplier and insisting on batch-specific data saved months and pushed them to upgrade their purification. After that experience, I checked every incoming bottle. Others in the lab got into the habit too, spotting oversights before projects fell off track.
Stronger standards don’t just happen—customers need to demand them. If a supplier can’t offer full spectra and openly discuss their purification steps, it’s worth spending a bit more with a competitor who does. Regulatory bodies like ISO set frameworks, but hands-on vigilance and transparency at the buyer level drive real change. Choosing suppliers who value purity and back up claims with data sets the right expectation for everyone downstream.
Takeaway for Buyers and Researchers
Whether you’re ordering a gram or a kilo, check the purity data before clicking ‘buy.’ Don’t take generic terms at face value. Look for suppliers willing to be grilled about their process—and be ready to pay for reliability. Real expertise shows up in both the paperwork and the final results you see in your lab.
Why Storage Conditions Matter
Maintaining the right storage conditions isn't just a line on a label. Think about the last time a bag of flour collected moisture in your kitchen—clumps, spoilage, sometimes a sweet invitation for bugs. Storage isn't just about keeping things tidy; it's about preserving value and ensuring safety. In my years of trying to stretch household budgets, nothing stings quite like tossing out good food or supplies all because the basics of storage slipped our minds.
Temperature Means More than Convenience
Heat and cold play a constant tug-of-war with most products. For food, the fridge and pantry choices can mean the difference between a fresh-tasting meal and a science experiment gone bad. Medication stories are similar; there’s a reason insulin vials need a cool spot, away from sunlight and heat. Even paint cans or DIY supplies, left in an overheated garage, clump up and settle. Reports from the Food and Drug Administration show that medication efficacy drops when ignored storage instructions lead to chemical changes or contamination. High humidity and warmth encourage bacteria, mold, and loss of product strength.
Why Air, Light, and Seals Make a Difference
There's a big gap between a tightly sealed jar and an open box shoved in the back of a cabinet. Even the smaller things, like that cardboard carton of salt getting rock-hard from steam and air, build frustration. My grandparents swore by airtight containers for a reason. Exposure to air dries out, clumps, or even degrades products. Light accelerates decay, especially for items with vitamins or active ingredients. For households with kids, accidental contamination can pose a real health risk—think of the stories of little ones getting into grandma’s open vitamin bottle.
Following Labels Isn’t Just For Show
It’s tempting to skip label instructions. Busy days encourage shortcuts. But clear guidance comes from years of research, not guesswork. For example, dairy kept just a few degrees too warm grows bacteria rapidly. New packaging technologies aren’t just a marketing gimmick. Some are designed to let air out and keep moisture in check, showing growing awareness about contamination and freshness.
Spotting Problems and Fixing Storage Gaps
Keeping food and products in order takes regular checking, not once and done. I once lost a whole set of winter vitamins from leaving them in a steamy bathroom cabinet—rice packets in humid kitchens have clumped beyond hope. A few basic rules pay off: dry, cool, dark spots do more for most household products than fancy storage bins. Rotate stock, check for late expiration dates, seal everything that comes in a bag, and elevate products off direct floor contact during wet seasons. If something changes smell or texture, don’t gamble.
Supporting Facts and Everyday Solutions
Research from the World Health Organization indicates up to 30% of global food waste happens from poor storage. Foodborne illness data ties spoilage to mild mishandling at home, not just in stores. Smart home habits—like using silica packs in sealed containers or transferring flours and grains to glass jars—can stretch budgets and keep families healthy. Sometimes, the solution isn’t expensive. A simple reminder to read storage details or a regular sweep of the pantry keeps costs down and spirits up.
Many Industries Run on Scale
Factories and commercial plants don’t stop at a few bags of material. In my years walking through warehouse floors, talking to purchasing managers, and visiting production lines, it becomes clear that buying in bulk is not a perk—it's the only way large operations keep margins sharp and supply lines reliable. Construction firms, manufacturers, and processors all look for solutions that come in drums, totes, or truckloads. They want a steady stream that keeps their production machines running without the risk of shortages.
Challenges Companies Face with Bulk Orders
Some companies hit a wall with product suppliers who deal only in small-scale quantities. If you’re a paint manufacturer, daily drum deliveries of chemicals aren’t enough; you need tanker trucks. A baker needs pallets of flour, not 5-pound bags. Securing consistent supply often involves negotiating contracts, keeping a finger on shipping routes, and keeping up with market swings that hit raw material availability.
Costs add up quickly when suppliers charge premium rates for smaller shipments. Bulk buying typically trims costs per unit. This makes a real difference on big projects where thousands of dollars can ride on supply chain efficiency. Less packaging waste also means fewer headaches for companies seeking sustainability certifications or striving to reduce their landfill footprint.
Quality, Consistency, and Trust
It’s easy to find single boxes of product online, but scaling up raises questions. Companies don’t just grab quantity; they also count on repeatable quality. Over my conversations with plant managers, they worry about product variation from different batches. Any slip in formula can ruin a production run. Established suppliers who understand the importance of batch consistency and clear lot tracking often win loyalty from businesses year after year.
Technical data sheets and thorough safety documentation matter a lot more at an industrial scale. Teams look for transparency, ways to verify specs, and a partner who stands behind their shipments if things go sideways. Reports of delayed shipments or questionable substitutions push purchasing managers to look elsewhere.
Practical Solutions for Easier Bulk Sourcing
I’ve seen companies flourish by forging direct supplier relationships. Negotiating for weekly bulk deliveries, setting up automatic reorder schedules, and keeping close communication with warehouse staff often makes all the difference. Some businesses use procurement software to compare real-time pricing and inventory, sidestepping old delays from back-and-forth emails.
Product traceability is non-negotiable now, especially in industries like food, pharmaceuticals, or electronics. Reliable bulk suppliers offer robust traceability systems, from batch codes to supply chain verification. Global companies often select vendors who pass third-party audits and offer ISO certification or similar credentials.
Local sourcing isn’t always the cheapest route, but the reduced risk of international shipping snags can tip the scales for time-sensitive projects. Sometimes, pooled buying groups help smaller factories access the deals usually reserved for industry giants. This kind of resourcefulness helps level the playing field.
What Matters Most for Industrial Buyers
Above all, buyers want access to supply partners offering large quantities without sacrificing QC standards or support. They expect up-to-date compliance paperwork, professional logistics, and a product that’s been through hands-on testing under real-world conditions. Experienced industry players look for stability—hoping their trusted supplier today will still be delivering next year, through both steady growth and the occasional market jolt.
Understanding the Chemical’s Hazards
1-Carboxyethyl-3-Methylimidazolium Chloride, often lumped with ionic liquids, shows up in a handful of specialty lab and research settings. Its job can range from acting as a solvent to facilitating certain reactions that regular water or alcohol won’t accommodate. Don’t let the long name distract from a simple fact: this isn’t something anyone wants spilled on their skin, eyes, or bench. The safety data reveals that it’s corrosive. Direct skin or eye contact leads to burning, redness, or worse. Breathing in small particles causes coughing or throat irritation. For those of us who’ve cleaned up a chemical splash before, that burning sensation sticks in the mind far longer than any spreadsheet.
Real-World Experience with Safer Workspaces
Spending years in college and industry labs, I’ve watched younger staff underestimated chemicals that didn’t smell strong or didn’t come with the “danger” symbol everyone recognizes. Small volume doesn’t mean tame. With this chloride, personal protective equipment (PPE) stands as the first line of defense. Nitrile gloves, lab coats, and chemical splash goggles should get worn every single time, not just when the supervisor walks by. A fit-tested respirator belongs in the equation if dust or mist will form. The temptation to skip gloves for a “quick weigh-out” fades away the first time you see the results of accidental exposure during an incident report review.
Smart Storage and Spill Prevention Matters
Ionic liquids bring a certain reputation for being less volatile than older organic solvents, but that doesn’t mean safe handling stops there. Keep this compound in tightly sealed glass or plastic, away from food prep areas or break rooms. I’ve seen cross-contamination happen because someone thought a small label meant a small risk. Avoid keeping acids, bases, and strong oxidizers on the same shelf. Unintended reactions don’t just ruin experiments; they damage health and equipment.
Dealing with Spills and Exposures
Even the cleanest lab teams run into spills. Have a chemical spill kit ready—absorbent pads, neutralizing agents picked for acids or bases, heavy nitrile gloves, goggles, and an apron. If the liquid lands on skin, flush the area immediately with cool running water and remove contaminated clothing. Nothing beats fast action during those first moments. Eyes need at least fifteen minutes under the eyewash station, and anybody who inhales vapor or dust should leave the area and seek fresh air. Emergency protocols aren’t just there for the audit; they’ve prevented long-term harm plenty of times in labs I’ve worked in.
Safe Disposal Practices
Disposal deserves careful attention. These chemicals don’t go down drains. Contact hazardous waste professionals or the lab's environmental health and safety office for collection and disposal. The temptation to “just wash it out” causes downstream problems for water treatment plants and wildlife.
Promoting Awareness and Training
Few safety measures get stronger than ongoing training sessions and open conversation about near-misses. Chemical producers and safety organizations publish excellent guides, but no handbook replaces a team that takes shared responsibility seriously. I’ve learned more from five-minute huddles after close calls than from thick manuals. Good habits, proper labeling, and a culture that values safety above shortcutting the process keeps everyone heading home healthy after the research ends.