1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate: In-depth Commentary
Historical Development
The journey of 1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate started in the broader context of ionic liquids, a field that picked up steam in the late 20th century. Researchers chased alternatives to traditional solvents, eager to cut out volatile organic compounds both for worker safety and environmental health. A few decades back, imidazolium-based ionic liquids stood out for their stability and low vapor pressure, offering scientists a new toolbox. By the early 2000s, more labs started tweaking the imidazolium ring, realizing small changes could build entirely new applications. The introduction of functional groups, like carboxyethyl, marked a turning point, helping bridge the demands of chemical synthesis with green chemistry principles. Today, 1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate reflects years of iterative discovery—work marked by collaboration between academic research and chemical industry development, motivated by real-world need for safer, smarter, and cleaner processes.
Product Overview
This compound belongs to the family of room-temperature ionic liquids. Chemists pay close attention to its pairing: a carboxyethyl-methylimidazolium cation with a hydrogensulfate anion. That unique marriage gives it solvent power but with a green profile, supporting roles from catalysis and separation technology to analytical chemistry. Over time, practical experience showed its value beyond the lab–from waste treatment systems aiming to minimize secondary pollution to pharmaceutical synthesis seeking reliable reaction media with low toxicity risks. As customers grew to trust its handling characteristics, vendors met increasing demand for high-purity batches and clear technical support, signaling the move of this niche discovery into everyday chemistry.
Physical & Chemical Properties
1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate shuns the volatility common in old-school organic solvents. It flows as a clear or pale-yellow liquid at room temperature, boasting thermal and chemical stability under ambient conditions and moderate heat. Density typically falls in the range of 1.2 to 1.3 g/cm3, and since it’s ionic, the electrical conductivity holds steady, a feature valued in electrochemistry settings. Hydrophilicity runs high—this material mixes readily with water and polar organics while shunning non-polar solvents. Viscosity can increase in dry conditions, slightly limiting high-throughput mixing at industrial scales, yet improvements in formulation technology continually address these tweaks. The compound resists decomposition under ordinary atmospheric conditions but breaks down in the presence of strong bases or oxidizers. Thanks to the carboxylic acid group on the cation, the molecule also participates in hydrogen bonding, expanding its usefulness in extraction and catalysis work environments.
Technical Specifications & Labeling
Producers label containers with CAS numbers, batch identifiers, purity—frequently above 98% for research use—and precise storage instructions. Material safety data sheets spell out storage requirements, such as a cool, dry spot, away from sources of intense heat or incompatible agents like strong acids and bases. Common package sizes vary from small laboratory ampoules of a few grams up to multi-kilogram drums destined for pilot plants or production-scale applications. Most suppliers include batch-specific analysis showing residual moisture, halide content, and manufacturing trace impurities. Clear labeling allows traceability for regulatory review, a rising priority as industries align with green chemistry standards and stricter international shipping regulations.
Preparation Method
Synthesis starts with an alkylation of 1-methylimidazole using chloroacetone, leading to a 1-carboxyethyl-3-methylimidazolium chloride intermediate. This intermediate undergoes ion exchange or direct metathesis with sodium hydrogensulfate, yielding the hydrogensulfate salt. Operators report that careful control of reaction temperature and slow addition of acids help avoid side reactions and high salt byproducts. Vacuum drying and filtration remove volatile impurities and unreacted starting materials. The standard lab protocol scales directly for commercial production; a well-optimized process reduces waste and simplifies purification, reflecting the shift toward greener, less resource-intensive chemistry in manufacturing.
Chemical Reactions & Modifications
Functionally, the carboxyethyl group opens doors in derivatization chemistry, attaching performance-boosting moieties or facilitating immobilization on solid supports for catalysis. The hydrogensulfate anion plays its own part as a mild Brønsted acid, so this ionic liquid reliably supports acid-catalyzed organic transformations without introducing corrosive mineral acids. Over recent years, teams have tailored the core imidazolium structure, varying side chains to tune viscosity or hydrophilicity and swapping anions for other functionalities. These modifications shape the compound’s utility across new synthetic routes and custom material formulations, regularly appearing in patent filings and journals dedicated to sustainable chemical process design.
Synonyms & Product Names
Lab catalogs and academic articles refer to this compound under names including “1-(2-Carboxyethyl)-3-methylimidazolium hydrogen sulfate”, “CEMIM HSO4”, and “carboxyalkylim IM ionic liquid”. CAS registry and regulatory filings help standardize these synonyms, minimizing confusion in international communication and trade. Producers sometimes brand their formulations based on cation purity and isolation method, further emphasizing product quality for high-performance applications. Names may vary slightly in published studies, so researchers double-check registry numbers before ordering or replicating experiments, an essential part of accurate reporting and reproducibility.
Safety & Operational Standards
Direct exposure rarely triggers acute toxicity, but precaution matters. Workers keep the material away from skin or mucous membranes, as mild irritation can follow after long contact. Safety data sheets recommend standard PPE—nitrile gloves, chemical splash goggles, and lab coats. Ventilation systems capture aerosols during bulk transfer or stirring, and spill kits with neutralizing agents remain ready for accidents. Storage in tightly sealed, labeled containers avoids contamination and extends shelf life. Waste streams carrying the ionic liquid get routed through approved neutralization and disposal steps, aligning with local environmental and safety regulations. Training covers both handling and emergency response, especially as adoption increases outside traditional research settings into large-scale industry.
Application Area
Analytical chemistry teams use this material as a solvent for sample preparation in metabolomics and proteomics workflows. It supports solubilization of bio-organic molecules without protein denaturation—crucial for extracting sensitive analytes. In chemical synthesis, its acid character facilitates esterification and transesterification reactions, supporting production of fine chemicals, active pharmaceutical ingredients, and specialty materials. Environmental engineers value it in extraction protocols for heavy metal pollutants, using its tunable polarity to selectively pull contaminants from aqueous waste streams. The physical stability and ionic makeup give process engineers confidence for use in high-temperature or electrochemical operations, where regular solvents break down or vaporize.
Research & Development
Ongoing R&D centers on improving recyclability and reducing synthesis costs. Scientists investigate structure-activity trends that shape solubility and reactivity, targeting even greener process metrics. Collaborations between universities and industrial consortia push for scalable methods and life-cycle analysis of ionic liquids from cradle to grave. Innovation runs high in immobilized catalytic systems, where enhanced ionic liquid support improves yields and limits leaching. Detailed spectroscopic and computational studies explain why certain functionalizations boost selectivity or reduce side-product formation, giving rise to smarter design templates for new ionic liquids. Active patent landscapes around process innovation signal a healthy future pipeline for derivative products and applications.
Toxicity Research
Initial studies point to low acute toxicity for most ionic liquids of this class, but the long-term and environmental effects get attention as production ramps up. Ecotoxicological surveys monitor aquatic fate and bioaccumulation potential, guiding disposal and spill management plans. Comparative studies measure cytotoxicity on cultured human and animal cell lines, as well as mutagenicity and biodegradation rates. So far, results suggest this compound poses lower risk than many legacy organic solvents, but open questions remain regarding chronic exposure and breakdown products. Regulatory bodies watch closely, incorporating new findings into evolving safety frameworks. Responsible labs apply precaution, screening alternatives as research expands into new use cases and higher production volumes.
Future Prospects
Innovation in the field of ionic liquids marches forward, and this compound stands to serve a growing need for high-performance green solvents. Market demand follows stricter air emissions policies, and customers expect materials that won’t compromise process safety or environmental compliance. Scaling up often brings price reductions and unlocks new applications in areas like energy storage, sustainable separations, and next-gen manufacturing. Chemists continue to tweak the structure for better stability, recyclability, and performance under extreme conditions, confident that the next improvement will open entirely new doors. Each success feeds back to boost confidence in ionic liquids, shaping tomorrow’s industry standards and elevating both environmental stewardship and technical performance in specialty chemical sectors.
Why This Ionic Liquid Matters
Chemistry sometimes seems locked up in labs, but anyone watching the shift toward cleaner industry or new materials can see why some lab discoveries change daily life. 1-Carboxyethyl-3-methylimidazolium hydrogensulfate sounds like a mouthful, but it stands out as more than just a chemical curiosity. Its main value lies in being an ionic liquid, which means it’s a liquid salt at room temperature. These liquids don't evaporate fast and they behave differently than water or oil: that opens plenty of doors for scientists and engineers tackling real-world problems.
Pushing Forward Greener Chemistry
My experience working with catalysis projects in university research groups showed me how much waste traditional solvents create. This ionic liquid offers a path out. Industrial labs use it as a solvent and catalyst for chemical reactions. Unlike old solvents, which can pollute air or waterways, 1-carboxyethyl-3-methylimidazolium hydrogensulfate barely gives off vapor. It brings another benefit: after a reaction wraps up, scientists often retrieve and reuse this compound. Real tests in biodiesel manufacturing back this up, making burning less oil and sending fewer grams of carbon into the air possible.
Pulp, Paper & Recycling Get a Boost
Digging into how we process plants, 1-carboxyethyl-3-methylimidazolium hydrogensulfate shows its edge again. For years, breaking down tough plant fibers for paper or biofuels loaded landfills with toxic leftovers. Today, this liquid steps in as a pretreatment agent. It softens up cellulose and lets enzymes or other treatments work faster and cleaner. Studies by researchers at places like the Finnish Technical Research Centre show how ionic liquids help extract sugars and valuable chemicals, saving energy compared to older methods that use boiling acid baths.
Cleaner Metal Processing & Batteries
Sometimes, metals must be purified or plated. Old acids do the job, but generate piles of hazardous waste. I’ve read case study after case study on using this chemical for these steps, with far less cleanup needed. In battery development—critical for electric cars and solar storage—ionic liquids like this one bring stability and safety improvements over flammable organic solvents. Chemists at Argonne National Laboratory reported promising results replacing traditional battery fluids with ionic liquids like these, improving fire resistance and boosting charge/discharge cycles.
Where Do We Go From Here?
No chemical works without some caution. Large-scale use calls for a sharp look at the toxicity and cost. Compared to their fossil-fuel-based cousins, ionic liquids sometimes run more expensive or slow to biodegrade. But the promise outweighs the risks where pollution reduction or safer manufacturing means healthier workers and cities. Regulations can catch up, and researchers keep pushing to design versions that break down in nature or make recycling even simpler.
Tools like 1-carboxyethyl-3-methylimidazolium hydrogensulfate change the way industries think about waste, energy use, and the safe handling of harsh chemistry. Every new use case moves enterprises closer to a reality where production doesn’t mean more harm for people or the planet.
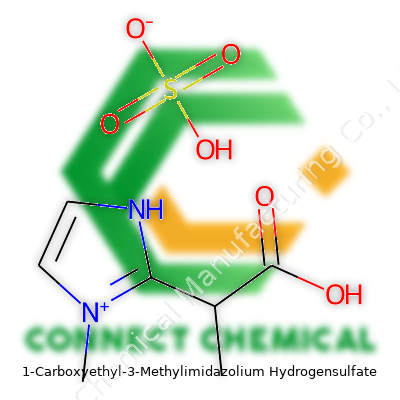
Molecular Structure and Chemical Formula
At its core, 1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate delivers a lesson in how chemistry keeps finding ways to solve real-world challenges. The structure starts with an imidazolium ring—think of a five-membered ring with two nitrogens at positions 1 and 3. Add a methyl group onto the first nitrogen and an extra piece called a carboxyethyl chain onto the third. This gives the cation part. The chemical formula for the 1-carboxyethyl-3-methylimidazolium cation lands at C8H13N2O2+. Pair it up with the hydrogensulfate anion, HSO4-, and the complete salt is C8H13N2O2+ HSO4-. For chemists, the detail matters: this arrangement makes a liquid salt known as an ionic liquid—something different from ordinary table salt, which stays solid unless heated way beyond water’s boiling point.
Why Structure Drives Function
Some folks might look at a chemical structure like this and shrug, but here’s where things get interesting. Imidazolium-based ionic liquids have shaken up both lab science and industry. Their unique structure offers strong solvation power for a range of substances—including stubborn organics and even metals. Imidazolium cations refuse to stay neutral; their charge and size push them into behaviors traditional solvents can’t match. Adding a carboxyethyl group increases hydrophilicity and can boost solubility for certain dyes, pollutants, or pharmaceuticals. Meanwhile, the hydrogensulfate provides acidity, which matters for catalytic reactions and extractions where pH control tips the scale between success and failure.
Real World Applications and Their Impact
Ionic liquids like 1-carboxyethyl-3-methylimidazolium hydrogensulfate started getting traction because classic organic solvents put health, safety, and our environment at risk. Volatile solvents spill, evaporate, and pollute. Ionic liquids don’t fly off as vapors. In my old lab, we spent more money on fume hoods and hazardous waste barrels than on actual experiments. These salts solve some of that because they barely evaporate and can handle high temperatures. Their chemical structure means you tweak the cation or anion and change their properties. That flexibility cuts down on toxic emissions, lowers fire risks, and opens new doors for energy storage, electroplating, and biomass processing.
Chemistry With Responsibility: Avoiding Blind Spots
The hype around ionic liquids needs a reality check. They’re not always biodegradable, and some stick around in the environment. That’s a problem ignored by marketing teams but not by people working on green chemistry. Carboxyethyl-3-methylimidazolium hydrogensulfate is a step forward, since the carboxy group can sometimes make the compound more water-compatible and less likely to bioaccumulate. Yet, relying on chemical tweaks instead of full life-cycle analysis risks repeating the mistakes of older solvent generations. Biodegradable variants and closed-loop recycling systems offer a route forward. Regulatory and peer-reviewed research must weigh real benefits versus unforeseen side effects. Transparent studies and open-access databases help anyone from grad students to CEOs make informed choices.
Finding Practical Solutions
Progress happens when lab research bridges to practical applications. If ionic liquids drive down pollution and raise process efficiencies, industry wins. Developing greener synthesis pathways for compounds like 1-carboxyethyl-3-methylimidazolium hydrogensulfate keeps waste low and makes recycling possible. Pilot projects that recover and re-use these liquids from chemical processes promise both economic and ecological payback. Collaboration between universities, startups, and established manufacturers pushes the field ahead. In the end, the molecular structure isn’t just about bonds and atoms—it’s about driving positive change, blending technical progress with responsible stewardship.
Staying Alert: Why This Matters
Every time I pick up a container with a warning label, I remember working in a small warehouse years ago. One day, a coworker skipped the gloves. He figured it would be fine for just a quick pour. It wasn’t. Skin contact with that cleaner left him with a nasty rash. Growing up, I thought warning symbols only showed up in science labs. Turns out, they often pop up around the house, the garage, and even at work. Missing or ignoring basic safety steps can lead to burns, breathing trouble, eye injuries, or worse.
Simple Steps, Serious Impact
Grabbing proper gear makes a world of difference. Gloves and goggles are top of the list. I once splashed a strong solvent onto my shirt, thinking cotton would protect my skin. Lost that shirt and spent days dealing with irritated skin. Even professionals run into trouble when they get comfortable and cut corners.
Ventilation matters too. Fumes don’t always smell. I learned this the hard way, fixing up my first car in a closed garage. Headaches crept in out of nowhere. Today, I make sure windows stay cracked or fans pull air outside. Respirators help in tighter spots. No one looks cool coughing through chemical dust or cleaning vapors.
Labels Carry More Than Legal Jargon
Reading instructions sounds basic. Many people rush through or ignore small print. Those lines often hide gems—like directions for mixing, storage tips, or ways to handle spills. Misusing a product—say, mixing bleach with ammonia—can make a toxic cloud. That can land someone in the emergency room. Before I open a new product, I scan the label right there in the aisle. I can’t count the number of times that habit kept me from grabbing the wrong stuff.
Common-Sense Storage Pays Off
I keep chemicals in their original containers for obvious reasons. A soda bottle full of weed killer looks harmless until a thirsty kid grabs it. At home, I stow away garden sprays and drain cleaners high up, away from kids and pets. At work, locked cabinets do the trick. No matter the place, temperature swings change how safe some products stay. Storing them as labeled, away from direct sunlight or heat, keeps everyone safer.
Cleanup: The Final Step
After each use, washing hands with soap and water gets rid of residue. I noticed a lot of folks grab a chemical, then eat lunch without thinking. Dirt and residue follow us from bottles and sprayers to the sandwich. Rinsing tools or containers before tossing them saves others who might handle the trash. Little steps now prevent big headaches or hospital trips later.
Solutions That Stick
Training helps, but habits stick better when everyone looks out for each other. In every shop or home garage, taking two minutes to remind friends, coworkers, or kids about gloves and goggles saves a trip to urgent care. As products keep evolving, companies could add bold pictures and quick warnings—simple, but helpful. Reporting near-misses or problems means others stay safer down the line. People and companies that phase out the most toxic stuff and push clear rules make it easier for everyone to work safely.
In my experience, vigilance and respect for these little steps keep harm at bay. Safety isn’t just for experts—it’s for all of us, every single day.
Looking Closely at This Chemical
Every lab and plant has its own storeroom stories. The bottle that leaks, the label that gets smudged, the odor that lingers longer than you'd like. 1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate stands out for its usefulness in catalysis and as a green solvent, but it doesn’t forgive sloppy handling. This ionic liquid, with its mouthful of a name, pulls moisture out of the air and doesn’t play nicely with the wrong materials. That’s not just textbook talk—that’s headache prevention in action. If the bottles live where humidity creeps in or where metal shelves corrode, you’ll end up dealing with ruined product or worse, safety incidents.
Temperature and Humidity Count
Humidity and temperature make or break the stability of chemicals like this. Open storage near an air vent spells trouble. I’ve seen glassware left too close to windows warp and weep over just one summer. So, storage works best below 30°C, away from direct sun or heaters. It has a habit of drawing in water, so if the cap isn’t tight or the stopper’s worn, you’re asking for contamination. Moisture causes hydrolysis—think degradation, wasted money, and experiments down the drain. Sealed, dry, and away from wide temperature swings make for good practice.
Container Choices Matter
I’ve gotten frustrated by cheap caps and incompatible plastics more times than I can count. For this compound, glass or HDPE containers with chemical-resistant liners keep things safe and easy to handle. Weak plastics or flimsy seals lead to leaks or slow but steady loss of quality. Never use hand-me-down containers that once held food or other chemicals. Cross-contamination not only risks lab results but personal safety too. Good containers cost less than treating a chemical burn or ruined research work.
Labeling Never Gets Old
Every bottle needs labels that stay put through spills, scrapes, and the routine washdown. Use solvent-resistant pens or print labels, and slap on hazard symbols and the name in clear, readable type. In my crowded storeroom, a missing label once kicked off an hour-long hunt for the right chemical. Keeping things clearly marked steers clear of confusion, especially if emergencies hit and outside help steps in.
Staying Vigilant with Storage Locations
Not every room works for storing chemicals like this. Store away from acids, oxidizing agents, and strong bases. This precaution doesn’t stem from lab paranoia—it’s rooted in real-life disasters. A careless placement once led to fumes that sent a colleague home with a nasty headache. Keep incompatible substances apart. Consider secondary containment trays for each storage shelf. Spills happen fast, and trays catch drips before they sneak across floors or down drains.
Regular Checks and Responsible Disposal
Once each month, eye every bottle, cap, and label. Check for cracks, leaks, or signs of escape. If a bottle’s past its best, don’t push your luck trying to use it up. Follow regulations for hazardous chemical disposal—never down the drain, never into the regular trash. Old, yellowed, or crusted product poses unknown risks. Responsible disposal keeps people and the environment safe.
Wrapping Up with Some Sense
Storing 1-Carboxyethyl-3-Methylimidazolium Hydrogensulfate without drama boils down to three things: dry containers, smart location, and a habit of checking labels and caps. Small choices make a big difference. Safe storage shows care—for the work, for the folks handling the stuff, and for the world outside the lab’s four walls.
The Landscape of Ionic Liquids in the Lab
If you’ve ever set foot in a laboratory, you’ve probably noticed the rising popularity of ionic liquids like 1-carboxyethyl-3-methylimidazolium hydrogensulfate. Chemists like their versatility and strong solubility, especially when working on green chemistry or more complex organic syntheses. But seeing something used often doesn’t always mean it plays nicely with every compound it meets.
What Makes Compatibility Tricky?
At its core, this chemical is a room-temperature ionic liquid with a stable imidazolium ring and a hydrogensulfate anion. Its structure seems stable, but every chemist learns not to put blind trust in labels like “ionic liquid” or “green solvent.” The underlying truth: performance depends a lot on who’s sitting at the table. You pour this liquid into a vial with water, and it mixes well—the typical story for many ionic liquids. The strong hydrogen bonding also means good results with polar organic solvents like methanol or ethanol.
Problems pop up with certain bases or strong oxidizers. If you try tossing sodium hydroxide or potassium carbonate into the mix, things get unpredictable. The hydrogensulfate doesn’t stay quiet, especially as the pH climbs or drops sharply. Chemistry doesn’t stop with dissolving—side reactions and decomposition often tag along. In my own bench work, I once tried to use this ionic liquid as a supporting electrolyte for a basic solution but ended up with unexpected precipitates. Turns out, ignoring acid-base behavior leads nowhere good.
Solvent Issues and Storage Risks
Not everything will dissolve happily in 1-carboxyethyl-3-methylimidazolium hydrogensulfate. Hexane, heptane, and other nonpolar solvents barely interact. You might see phase separation, and in practice, this gets messy fast. Industrial processes or academic labs hoping to blend this liquid with nonpolars for extraction or catalysis run into low yields and low efficiency. It’s not stubbornness—it comes down to the ionic nature and how it refuses to mingle with molecules that can’t share or break hydrogen bonds.
Some people underestimate storage compatibilities. Storing this liquid alongside reactive halides or strong acids can be a recipe for trouble. Sulfuric acid or hydrochloric acid often degrade the cation, leading to loss of solvent and release of unwanted byproducts. Manufacturers’ safety sheets recommend keeping it sealed, dry, and away from strong acids, making this advice worth repeating for anyone who handles it—even once. I’ve seen small labs mishandle these materials and quickly regret trying to cut corners.
Facts and Solutions
Research backs up these observations. A study from 2020 in the Journal of Cleaner Production reported high success when mixing this ionic liquid with cellulose or lignin, thanks to the polar nature and strong ionic interactions. On the other hand, pairing it with hydrocarbons brought almost zero solubilization. Room temperature stability seems impressive at first glance, but decomposition can set in above 120°C, especially when mixed with strong reducing agents. Compatibility isn’t just about physical mixing—it’s about how little side reaction you can tolerate.
Many chemists get decent results by slowly testing mixtures before scaling up. Pilot experiments, coupled with solid understanding of pH and basic ionic stability, give a better shot at compatibility. Never assume a successful reaction in an academic paper means smooth sailing with your own solvents or reagents at hand. Lab teams do better with a reference chart, careful procedural notes, and a mindset willing to backtrack if cloudiness or phase changes start appearing.
Final Thoughts on Practical Use
1-Carboxyethyl-3-methylimidazolium hydrogensulfate stands out for some tasks, but it won’t solve every solubility or reactivity challenge on its own. Trying too many combinations by guesswork wastes time and resources. Careful reading and real-world trial help find the right mix—trust for this liquid grows with every safe, successful experiment.