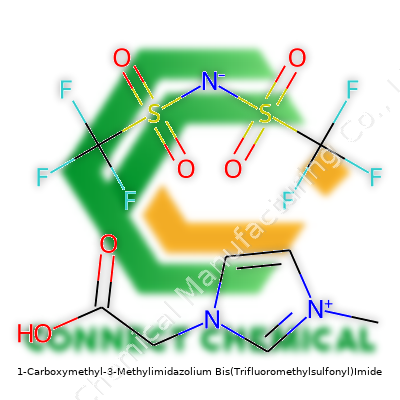
1-Carboxymethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide: A Deep Dive into Innovation and Impact
Historical Development
Scientists started exploring ionic liquids in the late 20th century, searching for alternatives to conventional solvents that could tackle pollution and safety concerns. Among the many, 1-Carboxymethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide rose in prominence. The imidazolium family drew attention for their thermal stability and negligible vapor pressure, drawing purpose from both environmentalists and industrial chemists. Over decades, tweaked chemical structures, better understanding, and shared expertise helped this compound move beyond labs into scale-up processes. Collaboration between chemists and engineers improved synthesis routes and impurity controls, marking a shift from academic curiosity to practical technology.
Product Overview
This ionic liquid showcases an imidazolium core flanked by a carboxymethyl group and a methyl group, balanced by a hefty bis(trifluoromethylsulfonyl)imide anion. This pairing grants not just chemical resilience, but also a steady performance across variable temperatures and pressures. The compound’s structure lends high ionic conductivity and resistance against hydrolysis, valued in electrochemistry and specialized extraction tasks. The fusion of carboxymethyl and methyl groups tailors reactivity and solubility profiles, accommodating plenty of research and industry settings.
Physical & Chemical Properties
Transparent to very slightly yellow in color, this ionic liquid usually appears as a dense, viscous fluid. It can remain stable at room conditions for long periods and retains fluidity below freezing temperatures, proving handy for low-temperature applications. Water solubility can vary with the purity and preparation method, but the carboxymethyl addition gives a hydrophilic edge compared to other imidazolium derivatives. Its low vapor pressure reduces emission risks, which makes it favorable in applications sensitive to flammability or workplace exposure. Strong resistance to acids and bases—along with its tolerance for repeated heating and cooling—connects to its use in batteries, catalysis, and electroactive devices.
Technical Specifications & Labeling
High-grade 1-Carboxymethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide typically claims purity upwards of 98%. Trace metals or halide levels, water content, and decomposition temperature feature in most certificates of analysis, aligning quality with regulatory and industrial protocols. Suppliers attach batch numbers for traceability and clear hazard pictograms to ensure safe shipping, especially since moisture or reactive impurities threaten its function. Packaging uses moisture-proof bottles—glass or Teflon-coated—because some plastics leach contaminants that alter its properties or shelf life.
Preparation Method
Manufacturers build the imidazolium backbone by condensing methyl imidazole with a carboxymethylating agent, often monochloroacetic acid or a related compound. Neutralization and metathesis reactions follow, swapping out counterions for the bis(trifluoromethylsulfonyl)imide anion. Strict pH controls matter, as do purification steps using activated charcoal or molecular sieves. Industrial scale-up means moving from batch reactors in glass to corrosion-resistant steel tanks, always watching for traces of residual halide, which degrade ionic conductivity and chemical reliability down the line.
Chemical Reactions & Modifications
Chemists often see the carboxymethyl group as a handle for further modifications, such as coupling reactions or esterifications, to make derivatives with tailored solubility or reactivity. This functional group allows the compound to grab metal ions or organic pollutants out of mixtures, supporting green chemistry initiatives and next-gen catalysis. Its stable anion permits work with oxidants and reductants that would wreck weaker salts. In labs, I’ve noticed how the core imidazolium body can withstand strong electric fields and support polymerization processes, letting people design batteries that stretch and bend, or separations that strip contaminants from water.
Synonyms & Product Names
Some catalogs list this compound under alternate names such as CMMI-TFSI or 1-(Carboxymethyl)-3-Methylimidazolium Tf2N. Global regulatory bodies assign CAS numbers and EC identifiers; scientists and engineers reference either for cross-checking data and ensuring shipment compliance. Producers sometimes abbreviate product names for ease, especially in electronic material supply chains where labels fill with acronyms. I’ve seen confusion from inconsistent labeling, which stresses the value of sticking to standardized terminology across purchases and research papers.
Safety & Operational Standards
Lab hands learn the importance of gloves and goggles with 1-Carboxymethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide, since accidental skin or eye exposure means a trip to the eyewash. At elevated temperatures, it might decompose to small amounts of corrosive or fluorinated off-gassing. Good practice means working under ventilation, storing the liquid away from strong acids or oxidizers, and logging any incidents. Material Safety Data Sheets advise against prolonged exposure and note that the compound’s low volatility doesn’t remove all inhalation risk. Facilities scope out spill response kits—special absorbents for ionic liquids—to avoid wastewater or soil contamination.
Application Area
This chemical finds its footing in electrochemical energy storage, supporting lithium-ion and next-generation sodium-ion battery research. I’ve worked on using it in dye-sensitized solar cells, where it can enhance both stability and efficiency. In catalysis, the compound acts as a green solvent—replacing volatile organics that choke up fume hoods and put workers at risk. Some engineers employ it in specialty separations for pharmaceutical production because it can select for specific molecules with impressive accuracy. The affinity of the carboxymethyl group grabs charged pollutants from industrial run-off, showcasing environmental potential. Much of the recent literature circles around sensors and actuators where mechanical flexibility meets chemical endurance.
Research & Development
Universities and corporate labs race to tweak this compound for broader utility. Project teams modify the side chains to enhance conductivity or reduce environmental impact, shaving off excess fluorine or cutting down synthesis steps for better green credentials. Patents track advances in the use as an additive for polymer electrolytes, shifting attention from straightforward solvents toward multifunctional active blends. I’ve sat in meetings where teams share pilot-scale successes and failings—real-world conditions flame out many theoretical designs quickly, so emphasis stays on operational robustness, affordability, and supply chain reliability. Grants reward approaches that demonstrate scale-up feasibility and less hazardous byproducts.
Toxicity Research
As new ionic liquids surge onto the market, toxicity insight lags behind product enthusiasm. Early studies on 1-Carboxymethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide point to low vapor inhalation risk and limited acute toxicity, but long-term environmental persistence remains unclear. Water organisms show some sensitivity; the heavy fluorinated anion part can bioaccumulate if not managed well. Labs now screen for chronic toxicity in mammals and aquatic life, alongside mutagenicity and breakdown pathways under sunlight or bacteria. Responsible suppliers limit shipments if toxicity thresholds remain unknown or if local environmental rules demand precaution. Robust research connects to genuine sustainability, balancing cutting-edge capability with practical responsibility.
Future Prospects
The momentum behind ionic liquids like this compound won’t slow soon. Commercialization tracks along the push for safer, greener chemical processes and advanced batteries that shrug off swelling and heat. With more companies demanding circular economies, researchers look at biodegradability and energy-efficient synthesis routes as must-haves. Smart surface coatings, adaptive gels, and wearable energy devices all represent frontiers for this material, tying chemistry to changing user needs. People on the ground weigh performance against cost, labor conditions, and environmental obligations—driving both conservative adoption and bold experimentation. The near future may see regulations pinning down disposal and recycling requirements, nudging R&D toward even cleaner, more transparent supply chains.
A Unique Chemical With Real-World Impact
Labs and factories rarely talk about 1-carboxymethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide over lunch, but this isn’t just another string of complicated words meant to confuse. People working with advanced materials, batteries, and green chemistry have been meeting this compound more and more. Instead of sitting in a dusty bottle, it’s pushing ideas forward in the real world.
Ionic Liquids in Action
This mouthful of a name belongs to a group called ionic liquids. Unlike table salt, which melts at over 800°C, ionic liquids become fluid below boiling water temperature. Think of cooking oil that carries a charge and can dissolve things water and oil can’t touch. I spent years in a lab searching for ways to make cleaner, safer solvents. You remember harsh chemical smells; ionic liquids help erase that memory. In research, this compound works like a silent, efficient partner. It doesn’t evaporate easily and shrugs off high temperatures, which gives scientists a stable environment for tricky reactions.
Cleaner Chemistry, Greener Choices
Anyone frustrated with waste from industrial processes should care about what goes down the drain. Ionic liquids like this one stand out because traditional organic solvents can pollute water and air, but this compound stays put. Companies making pharmaceuticals, plastics, and fine chemicals turn to ionic liquids because they help them meet tough environmental goals. A study from the American Chemical Society points out that using these liquids can slash hazardous emissions compared to classic solvents. There’s no magic bullet for pollution, but progress piles up drop by drop.
Electronics and Battery Development
Old batteries leak and degrade quickly. This compound shines in electrochemistry, especially in next-gen batteries and supercapacitors. I spent long nights watching battery electrodes swell and crack, only to find that ionic liquids gave them a longer, steadier run time. Researchers highlight this imidazolium salt’s wide “electrochemical window” – basically, it doesn’t break down under stress, so batteries run more safely and last longer. These details matter in electric cars and grid storage.
Catalysts and Separation Tech
Separation and purification are the workhorses in chemical plants. I’ve seen some setups rely on volatile solvents that risk fires or slow production. Swapping those out for ionic liquids can boost safety and sometimes help pull out precious metals or target impurities faster. Reports from chemistry journals mention how selective this imidazolium compound can be when grabbing specific ions or molecules out of a mixture, leading to higher recovery rates and lower costs.
Room for Growth
Adoption isn’t always smooth. Ionic liquids run pricier than some of the old-school materials. Toxicity studies, while promising, take time. Makers must weigh the benefits against upfront spending. Researchers keep digging for ways to recycle and reuse these chemicals, cutting down on waste and driving down expenses. Open discussion between industry, regulators, and academic labs will help break down those barriers and spread safer, smarter chemistry.
Getting to the Root of a Compound
Ever found yourself staring at a tangled chemical formula, thinking about what those letters and numbers reveal? The molecular formula cracks open the basics—how many carbon, hydrogen, oxygen, or nitrogen atoms you’re working with. It seems simple, but it can open a world of insight. Take glucose, for instance: C6H12O6 tells you the count, but not how the atoms connect. The difference between glucose and fructose, with the same molecular formula, comes down to structure. Tweaking even a single connection shifts how your body uses it.
Beyond the Letters and Numbers
High school chemistry taught a lot of us those ball-and-stick models, but they felt distant for the real world. As you look closer, though, structure drives how molecules behave. Acetaminophen and phenacetin offer a good example. Both pain relievers carry similar formulas. Still, their arrangements in space decide if a person gets relief or risks small but real harm. Structurally speaking, phenacetin’s oxygen atom sits in a different place. Years ago, this led to real trouble—phenacetin stayed on pharmacy shelves until links to kidney damage emerged.
Safety, Health, and a Little Curiosity
Plenty of everyday decisions rest on knowing what a chemical really is. Walk down any grocery store and check cleaning products, food packaging, or vitamins. You’ll see cryptic names or even just a string of numbers. Take household bleach: sodium hypochlorite’s chemistry—NaOCl—tells you just enough to get cautious when mixing it with acids or ammonia. Past experience working in a hardware store left no doubt—mistakes happen fast. Someone poured bathroom cleaner straight into a bleach-filled toilet, and the fumes cleared out half the aisle. Stories like that stick with you and shape respect for that fine detail. It isn’t just academic: the structure decides what does the cleaning, what stays safe, and what turns dangerous.
Hidden Connections: Medicine and Materials
Drug design leans heavily on these details. Generic medicines often stir debate, but the real focus falls on their molecular twins. One small difference, and you’re facing a medicine that doesn’t dissolve, absorb, or heal the same way. In 1982, a tainted supply of Tylenol highlighted how easily trust collapses when a molecular difference creeps in—even if by accident. Steps to check the formula and the structure went up after that scare, and rightfully so. The story isn’t just about chemistry but about practical impacts in people’s lives.
Keeping Chemicals Honest
Reliable chemistry depends not just on test tubes and white coats, but on accountability. Regulatory agencies inspect formula reports for food additives and pharmaceuticals. Experience writing about product safety showed the value of double-checking these certificates. If an ingredient altered during manufacturing, even in minuscule amounts, it set off a review that could stop a product launch.
Building Habitual Skepticism
It comes down to habits and transparency. Whether it’s a new research chemical, the latest kitchen cleaner, or an over-the-counter medicine, knowing the formula lets people ask smarter questions. Those questions keep companies honest and raise the standard. A clear molecular structure protects consumers, supports innovative research, and grounds regulations that matter in practice. It might look like alphabet soup, but getting it right saves trouble, time, and sometimes lives.
Why Safe Storage Matters
Anyone who’s worked in a warehouse, a kitchen, or even a home garage knows that safe storage isn’t just some checklist item. A single mistake—like mixing paint thinner with bleach or leaving food out in a warm spot—can ruin a week, a room, or even a life. Even products you think you know well have their own quirks. Following whatever directions show up on a can or bag often gets treated as a formality, but experience tells a different story. One warehouse I worked in learned the hard way after a minor propane leak: secure valves and proper ventilation aren’t just nice ideas.
Understanding the Risks
Each product brings its own risks. Cleaning liquids might seem innocent, but turn toxic if combined. Industrial powders get in the air and cause breathing troubles. Batteries overheat if they’re stacked wrong or left in sunny spots. Even cans of soup can attract rodents if they aren’t put away neatly. It’s important to know what you’re storing: read the hazard warnings and look up the manufacturer’s guidelines. The trick isn’t figuring out if something could go wrong—it’s asking how to make sure it never does.
Safe Storage: Clear Steps Anyone Can Take
Start by keeping products in their original containers. Home labels wash off or peel, and old soda bottles look too much like regular drinks to trust as storage. Store items where kids and pets can’t reach. Lock cabinets make sense for most chemicals—nobody wants to learn the hard way after a toddler mistake.
Temperature also means more than you think. Chemicals break down in the heat. Food spoils faster in a pantry without enough airflow. In the summer, I saw an aerosol can explode in a parked delivery van—ventilation would have saved some scrambling, not to mention the cleanup. For flammable products, keep them away from open flames and direct sunlight. Oily rags belong in metal cans with lids, not crumpled in corners. Flammables and oxidizers only cause trouble next to each other, so store them far apart.
Personal Protection and Clean Up
Anyone moving products with warning labels should use gloves, masks, and eye protection. Accidents feel remote until the sting of a splash or the cough of a dust cloud. Spills deserve real attention: absorb with sand or cat litter, sweep up, and wash hands afterwards. It doesn’t take a big mess to trigger an allergic reaction or slip hazard.
Keeping Records and Improving Habits
Most places I’ve worked keep a log for product uses, expiration dates, and storage checks. It takes some effort at first, but it pays off—especially if a batch ever gets recalled or shows up on a safety bulletin. Regular checks catch problems like leaks or swelling containers before things escalate.
Trusting your eyes and nose matters too. If a container starts bulging, smells strange, or feels warm, don’t ignore it—move it somewhere safer and get expert advice if needed. Colleagues and family appreciate warnings and reminders. A quick group huddle about safe handling after a near-miss helped our crew avoid repeating mistakes. Good habits outlast even the worst day.
Why Purity Matters
Anyone who’s handled chemicals for lab work or industrial projects knows that purity isn’t just a detail on a spec sheet. Purity can make or break an experiment, disrupt a manufacturing process, or be the difference between a safe outcome and a recall. Purity in chemicals usually refers to the absence of unwanted traces and contaminants. Laboratories rely on high purity for reliable data, whether that’s in forensic science, environmental monitoring, or materials research. A glass of tap water still counts as water, but tiny amounts of metals or microbes make a huge difference to a lab technician running a sensitive reaction or a manufacturer mixing up batches of product.
My own experience in basic research taught me that small impurities tend to act like saboteurs. Years ago, working with a supposedly “pure” acid salt, the presence of less than a percent of another metal ruined the color in a synthesized pigment. After a bit of detective work, checking batch certificates and contacting suppliers, the mystery cleared up: what was labeled as 98% was just not good enough for our need. For industrial scale users, a 98% product can work for cleaning or some manufacturing uses, while a pharmaceutical plant or high-tech electronics lab often insists on purity levels above 99.99%. Anything less, and the results get unpredictable.
Looking Beyond the Percentage
Purity can get more complicated than a simple number. Take sodium chloride – table salt for the kitchen, but for a microchip manufacturer, nothing less than “semiconductor grade” will do. Impurities aren’t always the same from supplier to supplier. Specification sheets list common culprits, but it takes some experience to read between the lines and know whether a 99% batch means just sodium chloride with a bit of magnesium, or if there’s something like heavy metals mixed in.
Sticking with trustworthy suppliers solves a lot of headaches; so does asking for full certificates of analysis before buying. This isn’t just about paperwork. Some suppliers volunteer third-party lab data or include QR codes that pull up quality control info. Sometimes, even a slightly higher cost buys peace of mind, especially if one error could cost a company thousands.
Packaging Options: More Than Just Boxes
Packaging might seem simple, but it’s another place where small differences matter. Chemical products show up packaged in everything from glass bottles and sealed drums to lined sacks and metal cans. Packaging sizes often start as small as 100 grams or 500 milliliters—think lab or small company needs—and scale up to 25 kilogram bags, 200-liter drums, or even tanker trucks for regular users in industry.
In my early days managing inventory, I learned to appreciate why flexibility saved time and money. A chemist running small batches doesn’t want a fifty-kilo drum taking up bench space. Meanwhile, a concrete maker with daily usage prefers shipment by the ton. One mid-size supplier I worked with solved frequent breakage problems by switching from glass to high-density polyethylene for certain corrosive chemicals, cutting accidents by half and making disposal simpler.
Sustainability now drives packaging trends. Some companies try biodegradable liners or offer bulk return programs so drums come back to get refilled instead of landing in landfill. Often, hazardous chemicals demand UN-rated containers or special labeling to keep shipping above-board and legal.
Best Practices for Buyers
The challenge, especially for newcomers, is matching purity to application and picking packages that fit their workflow. It sounds basic, but double-checking purity specs directly with the supplier, confirming packaging meets handling and legal requirements, and thinking a step ahead about disposal and storage keeps surprises at bay.
From my work on both the supply and lab sides, clear communication between buyer and supplier solves more problems than any catalog description can. A quick phone call to clarify purity or packaging leads to the right fit faster than days of email back and forth.
Everyday Experiences Shape Our View on Safety
Over the years, working around chemicals has taught me that respect for a substance starts with a clear head and a good set of gloves. A faint smell, a little cloud of dust, or the way a bottle label looks faded—these details deserve attention. Sometimes a substance has a reputation for being “safe,” but a casual attitude causes accidents. I once saw a colleague suffer a burn from a bottle left without a proper cap. Even routine actions, like pouring from one container to another, can turn risky in a blink.
Common Hazards Found in Chemicals
Some chemicals bite the skin. Others go straight into the lungs if the fumes float up at the wrong angle. Flammable liquids can catch fire before anyone lights a burner. I remember reading a report from the CDC that found chemical accidents at home and work send thousands to the ER each year. Chlorine, ammonia, acetone, or strong acids—they all need extra care. Skin, eyes, lungs, and sometimes even stomachs face trouble when folks let their guard down.
Mixing certain cleaners, for example, sends up toxic fumes. Even a little spill on an ungloved hand may lead to hours or days of irritation. Chemicals that seem harmless sometimes hide long-term risks, including cancer, nerve damage, or even fertility issues. Stories from old factories or labs remind us what happens when safety slides. Old-timers wore masks and washed their hands because they watched co-workers get sick over time.
Reading Labels, Asking Questions
A quick scan of a Safety Data Sheet (SDS) delivers crucial info. I still remember the first time I saw the hazard symbols—a skull for poison, a flame for flammability, a test tube for corrosives—it sticks with you. Taking time to read before diving in saves more trouble than any quick fix. No matter how familiar something feels, chemicals earn their warnings.
I keep a habit of asking how much ventilation is enough and if the goggles fit right. Communicating with coworkers or others in the house keeps everyone on the same page. Sometimes folks assume a splash won’t matter, but eye injuries from even a drop can last a lifetime. The importance of fresh air, the right gloves, and proper storage can’t be shrugged off as extra steps.
Practical Solutions Made Simple
One basic move—never storing chemicals near heat or open flames—has prevented countless fires. Double-checking labels keeps bleach from mixing with ammonia. Investing in sturdy gloves and reliable goggles prevents more visits to the doctor than any insurance card. Ventilated workspaces, closed containers, and good habits form the backbone of chemical safety.
In schools, homes, and workplaces, training pays off. Stories about accidents grab headlines, but it’s often regular, small acts of caution that save the day. Simple choices—like cleaning up spills immediately and disposing of waste properly—keep everyone safer. Nobody learns these lessons in a vacuum; experience and clear instructions change the way we treat every bottle and jar. People who share their close-call stories do a service: they help remind others that chemicals, even the ones we use daily, deserve our full attention.