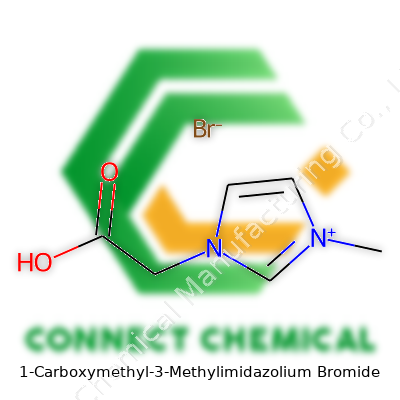
1-Carboxymethyl-3-Methylimidazolium Bromide: An In-Depth Commentary
Historical Development
I’ve watched the story of ionic liquids unfold over the past few decades with a mix of skepticism and respect. Back in the early 1990s, many chemists looked for alternatives to volatile organic solvents. Amidst this search, imidazolium-based ionic liquids began gaining traction, attracting keen interest for their tunable properties and practical applications. 1-Carboxymethyl-3-methylimidazolium bromide did not appear overnight; it is a deliberate product of smart design, a response to the real demands in catalysis, extraction, and green synthesis. Laboratories in universities and research institutions worldwide fed off one another’s curiosity, using incremental advances to push beyond simple imidazolium halides, engineering new anions and cationic structures to deliver more selective, safer outcomes.
Product Overview
1-Carboxymethyl-3-methylimidazolium bromide is more than some alphabet-soup chemical. For anyone working in coordination chemistry or electrochemistry, its value shows right up front—a water-miscible ionic liquid, typically a faintly yellow, viscous liquid or solid under standard conditions. Product availability has grown as chemical suppliers responded to rising research demands, ensuring scalable synthesis and reliable purity. Anyone sourcing this compound knows the frustration of inconsistent labeling and purity; the more recent surge in supplier competition brought cleaner, better-characterized material for both academic and industrial labs.
Physical & Chemical Properties
This salt packs a molecular punch with its balanced hydrophilicity, melting point in the 90-120°C range (depending on water content and trace impurities), and stable behavior under mildly alkaline and acidic conditions. The imidazolium ring loves to hydrogen bond and pi-stack, so handling requires attention to humidity and air exposure—water soaks up fast and may shift its appearance from a powdery solid to a sticky amass in hours. The bromide counterion creates pronounced ionic conductivity, which matters if you’re designing a system that handles current transfers or requires electrochemical robustness. The salt dissolves cleanly in water and methanol, opening doors to catalytic and separation work in green chemistry.
Technical Specifications & Labeling
For buyers and lab managers, the tech specs mean more than a number in a COA. Purity standards these days typically top 98%, and chromatographic methods confirm identity and assess contaminants. Most responsible suppliers now include full batch traceability, contaminant level summaries, water content (often by Karl Fischer titration), and an updated molecular weight calculation reflecting possible hydrate forms. These tidbits aren’t just bureaucracy: they make the difference between reproducibility and wasted weeks. Anyone in regulated sectors keeps an eye out for accurate labeling, proper GHS hazard symbols, and transport advice on the package.
Preparation Method
Running the preparation never feels like following a cake recipe. 1-Carboxymethyl-3-methylimidazolium bromide is usually synthesized by quaternization of 1-methylimidazole with bromoacetic acid in aprotic solvents, typically under inert atmosphere to protect sensitive intermediates from atmospheric moisture and unwanted side reactions. Yields have improved as process engineers refined control over stoichiometry and stirring rates. Post-synthesis, the salt often needs recrystallization from ethanol or acetone and multiple drying steps under vacuum to guarantee low water content. Anyone responsible for scale-up watches for runaway exotherms or bromide volatility, small reminders that chemistry rewards respect, not shortcuts.
Chemical Reactions & Modifications
Researchers who value functional customization come back to this salt for a reason. The carboxymethyl side chain invites esterification, amidation, and salt metathesis. Cation exchange allows preparation of customized salts for specific applications, including ion-pairing or templating in advanced materials. Electrochemical modification on the imidazolium ring expands its portfolio in sensors and redox applications. Bromide removal and exchange, for example with PF6- or BF4-, mark entry points for tailoring hydrophobicity or thermal stability. The carboxyl group, often activated using carbodiimide chemistry, paves the way for grafting onto surfaces or elaboration into more complex ionic liquid building blocks. The possibilities open space for personal creativity and scientific discovery.
Synonyms & Product Names
Chemists rarely stick with a single name. This ionic liquid pops up under plenty of labels, including 1-Methyl-3-(carboxymethyl)imidazolium bromide, MIM-CH2COOH Br, and in more proprietary blends as simply "functionalized imidazolium ionic liquid — carboxylic". These naming quirks reflect different supplier conventions and often confuse those new to the field. In the end, checking structural diagrams and CAS numbers before ordering ensures you avoid costly mistakes. Over time, those working closely with the material become fluent in its identity, making sense of catalog designations that once seemed scattered.
Safety & Operational Standards
Safety calls for respect, not paranoia. Even though this product boasts low volatility, bromide salts can cause discomfort on skin, eyes, and lungs, especially in fine powder form. MSDS documents stress wearing gloves, goggles, and handling within well-ventilated spaces or fume hoods. Waste disposal under bromide regulations prevents environmental build-up, an issue that lands hard on academic labs looking for green credentials. Quality assurance in manufacturing brings batch consistency, but users who test their own material for residues and water content avoid accidents. The rise of automation and enclosed transfer systems has further trimmed risks in bigger operations. Staff training makes the decisive difference—not checklists, but real experience with spills, exposure, and first-responder protocols.
Application Area
I’ve seen teams test this salt everywhere from biomass processing to pharmaceuticals. Its tunable polarity and charge distribution make it clutch in catalysis, extraction, and even as a medium for enzyme immobilization. In energy storage, conductivity and electrochemical window performance support development of next-generation batteries and supercapacitors. Analytical chemists use it as a modifier in capillary electrophoresis and liquid chromatography, aiming for sharper separation or cleaner baselines. As a green solvent, it replaces petroleum-based options with lower vapor pressure and better environmental persistence, though regulatory pushback often underscores careful disposal and lifecycle monitoring.
Research & Development
Teams worldwide approach this molecule as a gateway to broader families of functionalized ionic liquids, each with a new twist on performance. Labs spend months creating libraries of substitutions on the imidazolium ring; some yield better catalysis, others shine in anti-corrosion or antifouling coatings. Collaborative projects paint a detailed picture of solubility limits, thermal behavior, and ecological fate following disposal. Funding agencies are keenly interested in practical applications: researchers who can demonstrate improved process efficiency or lower environmental risks grab more attention. Every published paper becomes a stepping stone, encouraging fresh synthesis, computational modeling, and pilot-scale engineering efforts.
Toxicity Research
Anyone who handles chemicals for years takes toxicity studies personally. Early hope for ionic liquids rested on their reputed “green” label, but detailed studies soon surfaced on aquatic toxicity, chronic exposure, and persistence. 1-Carboxymethyl-3-methylimidazolium bromide, with its charged nature and partial water solubility, does not degrade as fast as traditional organic solvents; aquatic organisms can show stress at moderate concentrations. Some studies in zebrafish and daphnia suggest attention must go well beyond LD50 tables if environmental standards matter. Long-term cytotoxicity and environmental engineering studies signal that routine disposal into water streams poses genuine risk. Teams looking to promote “green” synthesis with ionic liquids need solid, published safety data in their funding proposals, giving reviewers and regulators confidence that real-world use outruns the hype.
Future Prospects
This molecule is not reaching the finish line any time soon. Trends in sustainable chemistry and renewable energy push demand for smarter, more forgiving solvents, and functionalized ionic liquids like this one are right at the front of those efforts. Scale-up and production efficiency already attract new investments, with some companies piloting continuous-flow reactors for large-volume campaigns. Advances in toxicological screening could someday deliver variants that retain function and safety by design, not by chance. Cross-disciplinary teams, from material science to environmental engineering, see this compound as part of a toolkit with growing possibilities: from biodegradable plastics to precision separation for high-value metals and pharmaceuticals. It won’t fix every problem, but as more data pour in, I suspect its story will keep shifting, with new uses, better risk management, and tighter integration into advanced manufacturing. The future belongs to those who balance opportunity with responsibility—something chemists, industrial engineers, and regulators all need to get right as they move forward.
Looking Closer at the Structure
1-Carboxymethyl-3-Methylimidazolium Bromide—quite a mouthful. It’s no ordinary compound from your high school chemistry set. The molecule builds its foundation with an imidazole ring, a five-membered ring with nitrogen at two spots. This family of chemicals keeps showing up in pharmaceuticals, energy storage, and green chemistry. The structure picks up extra flavor with a methyl group (–CH3) on the third position and a carboxymethyl side chain hanging off the first. The full name spells out the arrangement as clearly as a street address.
Draw this on paper and you’ll see: the imidazole ring is flat, with a methyl group jutting off the third carbon, and at the first nitrogen, a carboxymethyl group—essentially a -CH2COOH—branches out. Next to this complex cation sits a bromide anion. They stick together by ionic forces, much like the salt crystals sprinkled on dinner or the rust on a wet bike chain.
What Makes This Structure Notable?
Chemists look at this particular setup and spot utility right away. Imidazolium-based compounds have landed key roles in ionic liquids. Adding a carboxymethyl group doesn’t just give textbook variety—it causes real-world shifts. The acid group (COOH) tacked onto the molecule introduces water solubility and offers ways to tune reactivity. This extra handle lets scientists attach new pieces, cut down on unwanted reactions, and make the compound greener. Whether tinkering in a university lab or scaling up for an industrial reactor, that flexibility matters.
Bromide counterions bring another edge. Halides like bromide play into how the molecule stacks and fits with others. Bromide's size and charge distribution influence melting point and solubility, so swapping it out can give chemists valuable options without needing a major redesign.
Why Structures Like This Matter
Take it from years of working alongside academic and industrial researchers: molecules never just sit pretty in a bottle. The tweaks to shape and function matter in creating sustainable chemical systems. Ionic liquids built with structures like this one have cut down on hazardous solvents in industry. The World Health Organization and EPA have both flagged the dangers of volatility in solvents, noting the risks for worker safety and air quality. Ionic liquids step in, offering replacements with less evaporation and fewer toxic byproducts.
I’ve seen colleagues in battery research use imidazolium salts to boost ionic transport and stability. Power storage hinges on the careful dance of ions, and balance between the cation and anion affects not only performance but also safety.
Taking Steps Toward Responsible Innovation
Innovation always attracts trade-offs. Some ionic liquids still need improvements for toxicity and end-of-life treatment. Regulators and policymakers wrestle with how to push greener chemistry forward without handing industry extra costs or headaches. Coaxing safer molecules out of academic journals and into practice means open data, lively debate among peers, and plenty of cross-disciplinary engagement.
Investment in alternative counterions, recyclable synthetic pathways, and better process management will keep these molecules a step closer to commercial reality—and to broader benefits for society. Every structural change, no matter how small, brings us closer to chemicals that perform with fewer downsides.
Everyday Chemistry Quietly Driving Change
Chemistry in the modern world walks right alongside us. Specialty chemicals rarely get headline attention, but they play a big role behind the scenes. One good example is 1-carboxymethyl-3-methylimidazolium bromide, which many researchers and manufacturers have turned to in the hunt for safer, smarter tools. After working in research labs and speaking to colleagues in materials science, I have seen firsthand how chemicals like this step up where older, messier substances fall short.
This compound belongs to a family of imidazolium-based ionic liquids. At a glance, it doesn’t sound exciting. But its structure brings something special: it mixes readily with water, breaks down more cleanly than many solvents, and doesn’t release volatile fumes. For those focusing on sustainable chemistry, this has opened up several new directions.
Green Solvent for Cleaner Reactions
Most chemical reactions need a medium to happen efficiently. For decades, fossil fuel-based solvents like toluene or benzene have dominated. They work, but at a high environmental cost. By contrast, labs testing 1-carboxymethyl-3-methylimidazolium bromide as a reaction solvent discovered they could run processes at lower temperatures and lower pressure. Water solubility simplifies cleanup. Personal experience in an academic synthesis lab showed me how shifting toward ionic liquids dramatically reduced hazardous waste volumes, while keeping yields high.
Synthesis of drugs, dyes, and specialty polymers now benefit from this kind of solvent. Biodiesel production especially sees better separation when ionic liquids stand in place of traditional acids. Engineers monitoring outcomes have seen drops in byproducts and less post-reaction fuss.
Electrochemistry and Materials Science
Ionic liquids open doors in energy storage. Here, 1-carboxymethyl-3-methylimidazolium bromide stands out thanks to its ionic conductivity and thermal stability. Electrochemists working on batteries and capacitors have fed this substance into prototype devices, reporting less electrode wear and fewer side reactions. During a battery seminar last year, a materials scientist shared data suggesting these alternative electrolytes boost cycle life, with less swelling and leakage.
Polymer chemists also pay attention. Ionic liquids help build advanced materials, including polyelectrolyte membranes essential for fuel cells. The presence of the bromide ion makes them useful in electrodeposition, electroplating, and other processes needing precise ion transfer. Researchers in advanced coatings and corrosion prevention are tracking this shift closely.
Environmental Impact and Wastewater Treatment
Many manufacturing plants now chase greener routes for water treatment. 1-carboxymethyl-3-methylimidazolium bromide excels at extracting heavy metals and organic pollutants. In large-scale trials, it has cleaned up dye-laden water from textile operations and helped pull toxic metal ions from electronics industry wastewater. Because it does not evaporate or break down unpredictably, it keeps those contaminants locked up, making collection and recycling easier.
The truth is, chemicals like this have reshaped how engineers and chemists think about pollution control. I’ve watched groups move from skepticism to enthusiasm quickly after running pilot tests that shrink environmental footprints.
What Comes Next?
It’s not perfect. Building reliable supply chains and testing long-term safety in varied environments need more work. Still, from lab innovation to industrial rollout, 1-carboxymethyl-3-methylimidazolium bromide keeps proving its value. Let's keep watching its progress through new applications. Each small win in green chemistry brings a healthier world within reach.
Getting a Grip on Safety
Most folks don’t spend much time thinking about what happens to a chemical after it comes through the lab or warehouse door. Having spent long days in chemistry labs and talking with warehouse workers, I know rules aren’t just lines in a binder—they’re your insurance policy. Shortcuts with chemical storage are a recipe for ugly surprises, including accidents, lost inventory, and health scares.
Respecting the Basics for Chemical Safety
Temperature and humidity make or break the condition of most compounds. One slip—leaving a bottle out on a rolling cart in direct sunlight—can trigger decomposition, change the chemical’s potency, or even start a fire. Good storage means keeping things in cool, dry, shaded places, away from HVAC vents and radiators. It’s not about being fussy, it’s about staying alive.
Labeling has saved me more than once. A clear, up-to-date label with hazard symbols takes the guessing out. Walking into a storeroom, grabbing a bottle marked with the right signal word (“Danger,” “Corrosive,” or “Flammable”)—those labels stop bad decisions. The new guy might not know what tetrahydrofuran does, but he knows that red diamond means he needs gloves and eye protection.
Segregation Works—Don’t Mix Enemies
Chemicals can turn on you when kept near their worst enemies. I learned this quick: keep acids away from bases, oxidizers far from organics, and never let cyanides and acids share a shelf. Explosions and poison gas aren’t just stories to scare young chemists—they come from these dumb mistakes. Color-coded storage zones or cabinets reduce confusion and help everyone stay on the right side of safety.
Proper Containers: More Than a Box or Bottle
You get what you pay for. Cheap, flimsy jugs buckle under pressure from volatile solvents, spilling juice you cannot always see or smell right away. I’ve held plastic bottles that dissolved in their own puddle. Stick to containers recommended by the supplier—HDPE for corrosives, amber glass for light-sensitive chemicals, double-sealed bags for the nastiest powders. The right cap matters just as much. One loose lid turns a safe bottle into a fume bomb.
Eye on Routine and Records
Re-checking stock isn’t a one-time thing. I once found a bottle of ether that crystallized around the cap, showing signs of explosion risk. Frequent inspections catch bottles that leak, swell, or crust at the rim—these need removal right away. Digital inventory helps here; no one trusts a list taped to the wall. Get everyone to sign off after pulling out or storing a chemical, since accountability keeps hands honest.
Training Means More Than Videos
No policy in the world protects people who don’t know what to look for. Real-world training counts more than any email announcement. After every incident I witnessed, a hands-on session followed, covering spill drills, PPE choices, and storage maps. Respect for risk comes from personal stories, not a slideshow. Rotate staff through these regular refreshers—fresh minds forget the fastest.
Fixes and Final Thoughts
Budgets get tight, but shortcuts in safe storage can cost far more. It pays to spend on lockable cabinets, fireproof storage, and sensor systems. Document the basics, keep them in plain sight, and call in experts if questions pop up. It’s not just about ticking safety boxes; it’s protecting lives, jobs, and peace of mind.
Why Purity Matters in the Lab
Anyone who’s spent time in a lab understands how much purity shapes results. Chemistry, as reliable as it tries to be, gets derailed by contamination or variances. For researchers and engineers working with 1-Carboxymethyl-3-Methylimidazolium Bromide, purity isn’t an afterthought. This compound—used in ionic liquid synthesis, catalysis, and advanced material production—has wide-reaching effects if slightly off-spec.
What Commercial Purity Means
Most suppliers offer 1-Carboxymethyl-3-Methylimidazolium Bromide with typical purity around 98%. Reputable companies publish a certificate of analysis detailing water content, halide levels, and the percentage of the main compound, often checked by NMR and TGA data. Lots listed above 99% are available but expect a spike in cost and limited supply. For routine use, 98% covers a broad swath of applications in university research or pilot plant setups. Trace impurities below 1.5% include water, unreacted starting material, and metal cations absorbed during processing. This isn't window dressing. Minor components have triggered false positives in analytical runs or fouled expensive catalytic runs.
Why the Purity Isn’t Higher
Manufacturers stay at 98% for a reason. Solvents, glassware, and raw inputs bring their own baggage. Attempts to remove every trace impurity through recrystallization or column work cut into yield, jack up the price, and often provide little benefit for most users. Overly ambitious purification can leave a sample with altered physical properties, impacting consistent results and raising headaches for scaling up.
Impact on Experiments
I've wrestled with impure batches of imidazolium salts before. Lesser grades played tricks—solubility didn't match the textbook, and conductivities hovered outside normal ranges. Cleaning up post-reaction took hours. I’ve seen a whole group lose a week’s work after unknown contaminants interrupted a sensitive catalyst screening. In contrast, a bottle with a full certificate of analysis gave us confidence. We understood not just what was in the bottle but how it might affect downstream chemistry.
Identifying the Right Grade
Suppliers sometimes market “analytical grade” or “research grade,” but these terms don’t always tie to strict numbers. Insist on certificates, not just marketing words. Credible outfits provide NMR spectra, show residual metals by ICP-MS, and illustrate water content—often under 0.5%. Seeing that transparency lets a buyer match the grade to the project. Synthetic organic chemists or battery engineers might demand over 99% for structure studies or electrochemical work. A teaching lab can settle for 97% if the work isn’t so sensitive.
Practical Steps for Quality
To avoid setbacks, review certificates from two or three different suppliers. Compare impurity profiles, not just purity percentage. If high-precision work lies ahead, request a sample and run an independent NMR or mass spec for peace of mind. For anyone in charge of tight timelines or budgets, keep a backup purification plan—recrystallize if a batch underdelivers. Check storage protocols, since ambient moisture creeps in if bottles sit open.
Improving Purity Moving Forward
Suppliers innovate slowly, but those open to feedback can tweak synthesis or packaging. Requesting better seals or inert gas flushing prevents water pickup. Greater use of green synthesis—fewer metal catalysts, less toxic solvents—leaves behind a simpler impurity profile. Labs that collaborate directly with manufacturers sometimes get custom runs at 99.5% at a premium. Building strong supplier relationships rarely leads to disappointment and avoids expensive troubleshooting during projects.
The Real-World Chore of Figuring Out Solubility
Anyone who’s mixed up a salt with water or chased a stubborn compound through different solvents knows there’s a gap between lab descriptions and the realities of the bench. I’ve stood over plenty of beakers, watching crystals stubbornly refuse to dissolve, and the claim that something is “soluble” means surprisingly little until you see it vanish into the solvent with your own eyes. 1-Carboxymethyl-3-methylimidazolium bromide lands right in the center of that practical battle between chemist and compound.
Understanding This Imidazolium Salt
The structure of 1-Carboxymethyl-3-methylimidazolium bromide brings together the imidazolium core, a carboxymethyl tail, and a bromide anion. It looks exotic at first, but the imidazolium group shares a long history as the backbone for ionic liquids. That’s meant these types of salts have been well-explored for their ease of handling, and thankfully their solubility profiles aren’t quite the wild card some other salts can be.
Solubility in Water: The Easy Win
Drop this compound into water and it goes into solution easily. Its imidazolium ring, decorated with a carboxymethyl group, boosts its affinity for hydrogen bonding. The polar nature of this salt loves the polar nature of water. Within seconds or minutes—temperature and stirring make the difference—the crystals give way and a clear solution forms. Researchers have leaned on this property. A study in the Journal of Molecular Liquids showed high water solubility for related imidazolium salts, supporting what I’ve seen myself.
With many ionic liquids, water can serve as both solvent and participant in the reaction mix. In synthesis labs, 1-carboxymethyl-3-methylimidazolium bromide plays well here; that makes it less of a headache during clean-up or when prepping for downstream reactions. Anyone who’s knocked over a flask knows the cleanup is radically easier when you’re not chasing after oily residues or half-dissolved solids.
Trying Organic Solvents: Not So Fast
Things shift quickly if you pour this salt into common organic solvents. Nonpolar options like hexane or toluene have zero interest in dissolving this imidazolium compound. Solvents like ethanol or acetonitrile do a bit better, but only if you use large volumes or heat things up. Even then, solubility can lag behind what water delivers.
I’ve found that mixing with strong polar organics like dimethyl sulfoxide (DMSO) moves the needle more, but practical use drops off unless the whole process needs that level of polarity.
Why Solubility Matters in Real Experiments
Pick the wrong solvent and a reaction can stall, precious chemicals get wasted, or chromatograms smudge up with tailing peaks. Being water-soluble opens up greener chemistry options and cuts down on waste. Troubles melt away when runoff or spills don’t end up with dangerous solvent residues that stick around. Plus, it makes the downstream isolation of products easier, saving money and time.
Looking Ahead: More Than Just Dissolving Stuff
For anyone designing reactions that call for ionic liquids or specialty salts, pushing for water solubility pays off. It’s usually safer, more sustainable, and often cheaper. There’s a push these days for “benign by design” solvents—the growing popularity of these imidazolium compounds fits right in.
If someone finds themselves forced to use organic solvents with 1-carboxymethyl-3-methylimidazolium bromide, reaching for a strong polar choice (like DMSO or DMF) makes sense, but there’s a case here for rethinking reaction pathways or sourcing more water-friendly analogues. Solubility isn’t just a checklist item in the specs; it shapes the whole workflow in the lab.