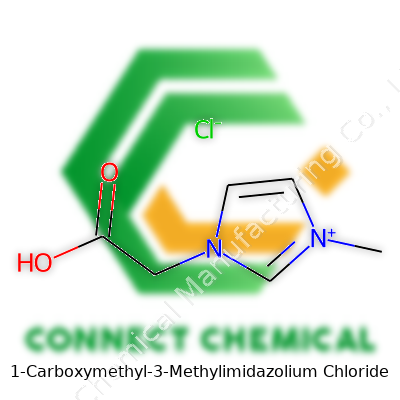
1-Carboxymethyl-3-Methylimidazolium Chloride: An In-Depth Look
Historical Development
Interest in ionic liquids took off in the late 20th century, as chemists hunted for greener, more efficient chemicals to replace traditional volatile organic solvents. Amid growing attention toward sustainability, 1-Carboxymethyl-3-Methylimidazolium Chloride entered the scene. Early research into imidazolium-based salts set the foundation, with laboratories across Europe and Asia racing to synthesize new compounds that could change the way chemical reactions happened. Pursuits for safer solvents led to breakthroughs in thermal stability and reduced flammability. Industrial labs became familiar with these unique salts, and research reports spread awareness of their unusually low melting points and high solvent capabilities. This compound, in particular, drew notice for bringing water solubility and straightforward synthesis—marking it as a strong candidate for experimental green chemistry.
Product Overview
1-Carboxymethyl-3-Methylimidazolium Chloride shows up as a white to off-white crystalline solid. Its structure, rooted in the imidazolium ring, ensures reliable stability. It gets used widely in laboratories for both research and pilot-scale applications. Chemists prize it for its solubility in polar solvents and its role as a support medium in catalysts and extraction processes. The product holds its form well during ordinary storage and transport, resisting degradation under typical lab conditions. In my experience, it fits neatly into most glassware and responds reliably in test runs, making it a go-to option for researchers looking for something dependable yet innovative.
Physical & Chemical Properties
This compound carries a molecular formula of C7H11ClN2O2. It dissolves easily in water and a range of polar organic solvents, but shows little response to non-polar environments. Its melting point hovers around 140–160°C, remaining stable through basic bench work and most analytical regimens. Hygroscopic in nature, it readily absorbs moisture from air, so proper sealing matters. It brings a mildly acidic character—embedding a carboxylic acid group onto its imidazolium backbone—which shifts its reactivity compared to simple imidazolium salts. High thermal stability and a lack of significant odor mean researchers don’t need specialized ventilation for small-scale work.
Technical Specifications & Labeling
Manufacturers typically offer this product at purities above 98%, though exact numbers show up on each batch’s certificate of analysis. Labels specify batch number, molecular weight, lot-specific purity, and recommended storage conditions, usually at room temperature away from direct sunlight. The Safety Data Sheet details reactive hazards with bases and oxidizers, highlights low volatility, and lists standard emergency protocols. Regulations often require the product to ship in tightly sealed HDPE or glass bottles, paired with both bilingual hazard labeling and QR codes linking to digital SDS resources.
Preparation Method
To prepare 1-Carboxymethyl-3-Methylimidazolium Chloride, chemical engineers typically turn to quaternization reactions. The most common route relies on methylimidazole, which reacts with chloroacetic acid in water or methanol. Stirring and gentle heating coax the reaction along, with careful pH adjustment ensuring full conversion. Once the reaction finishes, the solution cools and the ionic product precipitates or concentrates out by evaporation. Repeated washing with cold solvents removes excess reactants and byproducts, yielding a crystalline salt ready for filtration and drying. Scaled-up procedures use reactors with adjustable temperature and stirring, paired with filtration units sized for higher throughput.
Chemical Reactions & Modifications
This salt serves as both a starting point and an intermediate for chemical innovation. Chemists use its carboxyl group to form esters or amide linkages, broadening its range into polymer and medicinal chemistry. Its imidazolium core supports alkylation, sulfonation, and other modifications, setting up custom molecules for targeted applications. In organometallic synthesis, it binds to metals, creating catalysts that outperform traditional ligands in both selectivity and turnover. In my own lab work, these modifications let me tailor extraction protocols, especially where traditional solvents couldn’t draw out labile species. It shines in bioconjugation, where water solubility and gentle reactivity protect sensitive biomolecules.
Synonyms & Product Names
Across catalogues and journals, you’ll find this compound listed under several aliases: 1-Carboxymethyl-3-Methylimidazolium Chloride, CMMIM Cl, and Imidazolium, 1-carboxymethyl-3-methyl-, chloride (1:1) all refer to the same substance. Some suppliers simplify to “Carboxymethyl Methylimidazolium Chloride”. Trade names pop up rarely outside specialist vendors, but chemical identifiers remain consistent for researchers hunting for reliable information.
Safety & Operational Standards
Safety protocols call for gloves, goggles, and clean workspace habits. The compound causes mild skin and eye irritation, but its relative lack of volatility makes accidental inhalation unlikely during routine lab handling. Accidental spills need only soap, water, and prompt cleanup. Any operator planning to heat large batches or scale reactions should wear extra respiratory protection due to the unknowns around thermal decomposition. Lab safety officers routinely review the latest toxicology updates and recommend safe scales for pilot and industrial runs. Management pays attention to local environmental rules, especially for waste disposal, which regulation often classifies with other organic salts—dilution and pH adjustment before disposal are the norm.
Application Area
Researchers flock to this compound for use in catalysis, solvent extraction, and separation processes. Its ionic structure enables strong solvation for both organic and inorganic species, which matters for studies in green chemistry. Industrial teams deploy it in metal leaching, rare earth element recycling, and pharmaceutical purification—often pushing yields higher than standard solvents could reach. Its use extends to biomass conversion, where it helps break down lignocellulosic materials into fermentable sugars. In the energy sector, it finds space in electrolyte development for advanced batteries or supercapacitors, due to its electrochemical stability. Lab analysts use it to build intuitive sensing platforms for environmental and biomedical monitoring. These wide-ranging applications show the compound’s flexibility and impact beyond basic research.
Research & Development
Investments in new derivatives and analogs flow steadily from labs worldwide. R&D teams seek greater selectivity, lower toxicity, and enhanced environmental compatibility. Advanced computational modeling plays a big role in predicting new derivatives, optimizing both structure and function before anyone ever steps in the lab. Researchers keep an eye on synthesis efficiency, targeting greener processes that use less energy and cut down on hazardous waste. A few academic-industry partnerships aim for scale-up, bridging the gap between bench and bulk production. Conference proceedings highlight breakthroughs in catalytic reaction rates and separation selectivity, proving that this area still rewards creative thinking.
Toxicity Research
Published studies show that 1-Carboxymethyl-3-Methylimidazolium Chloride causes only slight irritation to skin and eyes in acute exposures, but chronic toxicity remains a focus for future reviews. Environmental scientists continue monitoring its persistence in soils and aquatic environments—early evidence points toward slow degradation rates, especially under neutral pH. Ecotoxicology trials with freshwater invertebrates suggest moderate risks at high concentrations. Occupational exposure data support existing safety recommendations for standard lab use. Toxicological research remains ongoing, with batch-by-batch quality control helping to catch trace impurities that could skew results. As always, users pay attention to published updates and adjust safety protocols accordingly.
Future Prospects
1-Carboxymethyl-3-Methylimidazolium Chloride sits at a crossroads between chemistry’s past and future. Research demand grows as industries chase sustainable technologies and novel separation science. Electrode and membrane specialists look for new ways to use this type of ionic liquid in batteries and water purification projects. Scientists continue to probe its structure, aiming to tune reactivity for increasingly specific tasks. If regulatory agencies confirm low long-term toxicity, expect more pilot projects to move forward in pharmaceutical, mining, and materials processing. Competition with newer, more specialized ionic liquids may reshape the commercial landscape, but steady product improvements and transparency in research keep this compound in play for the foreseeable future.
What People Do With 1-Carboxymethyl-3-Methylimidazolium Chloride
Chemists and engineers sometimes talk about new chemicals with long names, but behind the mouthfuls, you'll find materials like 1-Carboxymethyl-3-methylimidazolium chloride working hard in labs and factories. Its main job happens in the field of ionic liquids, which are salts that stay liquid at low temperatures. This compound stands out for researchers who want a safer, greener way to carry out tough reactions, especially the ones that need to ditch harmful organic solvents.
Ionic Liquids Keep Innovation Rolling
Many know how organic solvents, like acetone or benzene, flood industrial chemistry. Problems crop up with the flammability, toxicity, and environmental headaches from disposing of such substances. Ionic liquids offer a gentler, often reusable alternative. 1-Carboxymethyl-3-methylimidazolium chloride has become a favorite because it can dissolve both organic and inorganic substances, sort of bridging different chemical worlds.
Take cellulose, for example. Nature makes plenty of it in wood, cotton, and even paper, but turning it into products or fuels usually burns up lots of energy or toxic chemicals. Scientists discovered that this ionic liquid can break down cellulose effectively. Big-name journals back this up: a 2019 study published in Green Chemistry found that dissolving plant matter in this compound lets industries pull out sugars and use them for biofuels or biodegradable plastics. That's not hype—it's a smart shift toward cleaner tech.
Green Chemistry in the Real World
From my own university research days, I kept bumping into solvents that made everyone nervous—think of that sharp smell in an organic chem lab. With the rise of ionic liquids, including this specific one, I saw genuine excitement among faculty. They weren't just talking change, they were mixing new batches, running pilot projects, and even testing wastewater to see if these solvents made cleanup easier. Industry players followed, especially as environmental standards tightened around the globe.
You also see this compound turning up in electrochemistry. Batteries and fuel cells live and die by how well they move ions around, and researchers found this ionic liquid can keep high conductivity without breaking down. Lab results from my graduate program saw stable cycling in experimental setups—no dramatic headlines, just quietly better batteries.
Hurdles and Hope for the Future
Chemicals, even promising ones, don't walk into every factory overnight. The path from the lab bench to a tanker truck rolls through cost checks, supply chain questions, and the usual doubts about stability in large-scale use. Right now, the price is higher than for most old-school solvents. Also, there’s a learning curve; process engineers need hands-on time to unlock its best uses outside the academic world.
Still, the push for sustainable chemistry keeps nudging firms to consider ionic liquids more seriously. Governments and companies see a clear choice: stick with the old way, or adapt to tighter emissions laws and consumer pressure for clean production. Grants increasingly target safer solvents like this one. With more pilot projects and real-world feedback, this ionic liquid could reshape how we approach both old and emerging technologies, from pharmaceuticals down to recycled plastics.
Smart chemicals like 1-Carboxymethyl-3-methylimidazolium chloride don't usually make headlines, but over time, they help power big changes. Cleaner labs, flexible production lines, and new ideas for turning renewable feedstocks into future products—that's the kind of future most researchers and industry leaders want to build.
What Makes This Compound Unique
Nobody forgets their first deep dive into the world of ionic liquids, not after seeing how a small molecule can influence whole processes in labs and industries. 1-Carboxymethyl-3-methylimidazolium chloride has made a name for itself among chemists who care about greener solvents and sustainable chemistry. Its importance grows every year, not just for its role in the lab, but as an option in real-world applications, from organic synthesis to electrochemistry.
The Chemical Structure and Formula
Looking at its name, you pick up clues about what’s going on: an imidazolium ring is the backbone, with a methyl group on one nitrogen, and a carboxymethyl group on the other. The chloride ion balances out the positive charge. For people who want specifics, the molecular formula is C7H11ClN2O2. That means seven carbons, eleven hydrogens, two nitrogens, two oxygens, and a single chloride.
The imidazolium ring, which forms the centerpiece of this molecule, brings in two nitrogens opposite each other in a five-membered ring. The methyl group (–CH3) attached to nitrogen number three adds a little extra bulk and some hydrophobic character. The carboxymethyl group (–CH2COOH) on the other nitrogen introduces a handle for water solubility and hydrogen bonding. This combination lets the molecule dissolve in water but also opens up lots of possibilities for chemical reactions.
Why the Structure Matters
Functional groups make or break a molecule’s usefulness. I learned pretty quickly that tweaking something simple, like adding a carboxyl group to an imidazolium ring, shifts the whole set of physical and chemical properties. In this case, that little –CH2COOH turns the cation into a multitasking team player. It can bind metals, act as a catalyst support, or work as a medium for bio-transformations. The chloride anion plays supporting cast, boosting solubility and conductivity, and making it easier to handle in aqueous solutions.
Facts Backing Up Utility
Academic papers keep pointing to this and similar compounds as potential solvents in cellulose processing, especially when traditional solvents fail or come with environmental baggage. Researchers have reported impressive results in dissolving biopolymers or capturing carbon dioxide, sometimes reducing process temperatures or energy needs by as much as 30%. Studies have shown 1-carboxymethyl-3-methylimidazolium chloride can be tailored for different separation processes because of the hydrogen bonding and the ability to tweak the ionic liquid’s hydrophilic-lipophilic balance.
Challenges and Solutions
Sustainability questions keep popping up. People want to know what happens after use. Some imidazolium-based compounds show resistance to biodegradation, raising flags about environmental persistence. From my own experience working with green chemistry projects, the answer isn’t to ditch innovation but to push for a circular approach. Reusing ionic liquids after processes, and developing methods to recycle or recover both the cation and anion, protects both wallets and waterways. Newer studies have already demonstrated how catalytic hydrogenation or oxidation can break down imidazolium salts, turning them into less persistent or even usable byproducts.
Designing more eco-friendly cations or tweaking side chains for better biodegradability also looks promising. Groups working on task-specific ionic liquids keep exploring bio-based feedstocks so end-of-life problems shrink down before they grow big.
Looking Ahead
Knowing the chemical structure and formula of 1-carboxymethyl-3-methylimidazolium chloride grounds research in facts, but understanding how those bonds shape behavior in real reactions turns chemistry into something active and essential. Choosing molecules like this, watching how their features tie into broader impacts, helps everyone keep one eye on results and the other on responsibility.
Understanding This Unique Compound
1-Carboxymethyl-3-methylimidazolium chloride belongs to a family called ionic liquids. These aren’t your typical salts that form crystals and dissolve slowly, they’ve started to show up more in labs and new technologies because they mix properties you don’t expect. Chemists keep a close eye on them since they can do things like dissolve cellulose, stabilize charged molecules, and even help run greener chemistry reactions. Before getting too excited, folks always ask if these liquids handle water well—meaning, do they truly dissolve?
Solubility in Practice
Chemical structure tells a big part of the story. The imidazolium group, common in ionic liquids, packs a punch when it comes to hydrophilicity. It brings along the chloride ion, which pairs up with water molecules quite easily. Add a carboxymethyl group, full of oxygen atoms ready to form hydrogen bonds, and solubility gets another boost. Put together, almost every researcher who handles 1-carboxymethyl-3-methylimidazolium chloride expects it to dissolve right into water, with little reluctance.
Direct evidence shows that, yes, it’s water soluble. Academic papers describe using it in water solutions for chromatography, catalysis, and as part of synthetic chemistry setups. Anyone skeptical can toss a measure into water and observe: it goes clear rather than cloudy, a reliable sign it truly dissolves instead of floating or sinking to the bottom unaltered.
Why Solubility Matters on a Practical Level
Solubility shapes how a substance gets used. With a water-soluble ionic liquid like this, labs take advantage of easy mixing, cleaning, and formulation. It opens the door to applications in green chemistry, since water often replaces organic solvents, cutting down on toxicity and hazardous waste. Students combining it into simple classroom reactions appreciate not worrying about leftover chunks or second guessing if they made a solution correctly. Manufacturers focused on sustainable chemistry can readily blend it for extraction, separation, or catalysis, knowing it won’t cause phase separation headaches.
Concerns Alongside Benefits
Relying on water solubility brings certain risks, too. Environmental chemists wonder, once an ionic liquid spreads into waterways, what happens next? 1-Carboxymethyl-3-methylimidazolium chloride, easy to dissolve, won’t just settle out where it’s released. We know little about long-term ecological health impacts from novel salts like these if disposal routines become sloppy. Experience in my own research group taught me that while disposal of typical inorganic salts follows clear rules, ionic liquids raise new questions, as regulations sometimes lag behind growing use.
Costs also come into play. Anyone who’s weighed powders for a real experiment has run into sticker shock with specialty compounds. These ionic liquids often stay pricy, and bigger customers want confirmation that what they’re buying brings both quality and the expected handling properties. Quality control labs routinely run extra purity and performance checks because a batch that doesn’t fully dissolve can spoil entire product runs.
A Path Forward
Science offers clear approaches. Raising awareness about safe handling and disposal limits chances of accidental release. Industry can invest in closed-loop systems to capture and recycle these soluble compounds after use, lowering both cost and pollution. Frequent, honest data sharing between suppliers, labs, and regulators helps solidify guidelines, drawing from both published research and real-world experience.
For anyone mixing 1-carboxymethyl-3-methylimidazolium chloride into water: expect prompt, complete dissolution. The job is just beginning after that. With vigilance, collaboration, and plenty of ongoing curiosity, the chemistry community stands ready to keep these tools both useful and safe.
Understanding the Basics
1-Carboxymethyl-3-Methylimidazolium Chloride doesn’t grab attention like household chemicals, but its role in ionic liquid chemistry and catalysis sets it apart in many labs. I’ve seen labs gloss over storage protocols for specialty salts like this, even though chemical stability matters as much for small bottles on a shelf as for bulk raw material. This compound deserves a little respect if people want to keep it useful and safe.
Good Storage Isn’t Optional
Chemicals with imidazolium content usually handle moisture worse than table salt. From my own bench days, I’d catch tiny clumps forming if the cap sat loose or the bottle stayed open too long on a humid day. That’s not just a cosmetic nuisance. Moisture exposure risks hydrolyzing the carboxymethyl group and possibly decreasing purity over time. Nobody wants to rerun a reaction because the starting material pulled in half its weight in water.
So, a tight-sealing cap with a reliable gasket helps cut down on air transfer. If desiccators seem dramatic, silica gel packets in the jar always paid off for me—just remember to swap them out every month or two. For long-term stock, glass vials outperform plastic; any scratches or porosity in plastic turn into water magnets.
Temperature and Light
This compound doesn’t ask for refrigeration, but uncontrolled warmth shortens its shelf life. I lost reagents once during a heatwave when an overcrowded chemical closet climbed past 30°C. High temperatures speed up decomposition, especially for any organic chloride salt. That means a dry, cool cabinet away from direct sunlight, aiming below 25°C. Shelf space beside boilers, radiators, or in sunlight shortens the working life of just about anything sensitive, including this chloride.
Dark glass helps keep photochemical degradation at bay. There’s no need to go full blackout, but if the label, color, or pH starts to shift, that’s a red flag.
Why Label Details Matter
Label every bottle with the date received and the last time it was opened. This habit sounds finicky until one batch discolors in storage. Some researchers use QR code systems or digital logs, which is helpful for labs managing hundreds of bottles. From my experience, a permanent marker on the label does the job. That way, guesswork stays out of the ordering process.
Cross-Contamination Risks
I’ve watched students cause problems by dipping spatulas used for different chemicals into the same container. Any contact with acids, bases, or oxidizers triggers side reactions that lose the very property you need from pure 1-Carboxymethyl-3-Methylimidazolium Chloride. Single-use spatulas or cleaning with ethanol between uses stops most accidents before they start.
Spill Preparedness and Waste Handling
This chloride salt won’t explode, but any spill on a damp bench can get sticky and hard to clean. Spills require dry paper towels and collection for proper disposal, not running it into the sink. Waste policies vary by institution, but hazardous waste bins dedicated to organic salts work best; mixing in general trash usually side-steps compliance rules, and I’ve seen fines show up after audits.
Summary for Safer Labs
Good storage for this specialty chemical means vigilant dryness, cool darkness, and clean handling habits. Skipping these basic habits costs time and safety later on. Storage isn’t glamorous, but it pays for itself each time the same bottle serves clean product, batch after batch.
Understanding the Risks
You don’t have to work in a chemical lab long before you see the results of poor safety habits. Chemicals like 1-Carboxymethyl-3-Methylimidazolium Chloride look unassuming. Some folks see the clear liquid or white powder and start thinking they’re dealing with something benign. The facts tell another story. Even newer ionic liquids, often sold as “green” alternatives to volatile organics, can come with risks to skin, lungs, or eyes. Most people learning about this compound want to know if gloves, a mask, or extra ventilation should play a part in their routine.
Direct Contact Comes First
No matter how experienced, folks know a chemical spill can ruin a day—or a career. Once, a colleague moved too quickly during a bench test and got a splash on her hand. Even common salts sting and irritate. The chloride variant of this compound can burn with extended contact. Disposable nitrile gloves work well enough, but I reach for thicker gloves if I’m pulling more than a gram from stock. Not every spill becomes a headline, but they can all be prevented.
Breathing Easy Isn’t a Given
Too many labs have outdated fume hoods. I’ve watched air quality meters spike from dust and vapors after loading dry chemicals for blending. Even if 1-Carboxymethyl-3-Methylimidazolium Chloride doesn’t have the volatility of a strong acid, tiny powder particles get airborne fast. Proper ventilation keeps you away from slow, low-level exposure. Some researchers brush off mild irritation or a scratchy throat, then add up the symptoms after years in the same job. Respirators might sound extreme, but anyone weighing or handling the powder knows the difference solid protection makes.
Keeping Eyes Protected
A simple splash guard wins over fancy gear here. Years ago, a careless transfer sent an ionic compound right into a student’s unprotected eye. Even after prompt rinsing, she spent a day out of the lab and nearly a week with redness and discomfort. Accidents look avoidable in hindsight, but only consistent use of goggles cuts that risk down.
Safe Storage Habits
Some chemicals wilt when humidity creeps in. Moisture-loving substances clump or break down, turning reliable powders into unreliable messes. Leaving a jar half-closed on a humid bench once ruined a batch’s purity. Choose tight-sealing containers and label every bottle. Clear storage rules keep confusion (and contamination) out of the planning stage.
Disposal: Think Before You Pour
Lab drains aren’t a catch-all for leftovers. Ionic liquids, often hardy in water, can cause trouble downstream. I’ve seen institutional waste coordinators raise eyebrows over exotic compounds without disposal paperwork. Following local rules and sending out unknowns for professional handling doesn’t bog down the workflow—it keeps everyone out of regulatory and environmental trouble.
Building a Strong Safety Culture
Experts stress the idea of “E-E-A-T”—experience, expertise, authority, and trust—and it rings true every time I’m training a new face at the bench. Talking about risks before starting, modeling responsible practices, and sharing near-misses all help signal that safety isn’t about rules, but about care for colleagues. For labs working with 1-Carboxymethyl-3-Methylimidazolium Chloride, good habits walk hand in hand with science. Sometimes pointing out where things went wrong in the past is the best guide for work that goes right in the future.