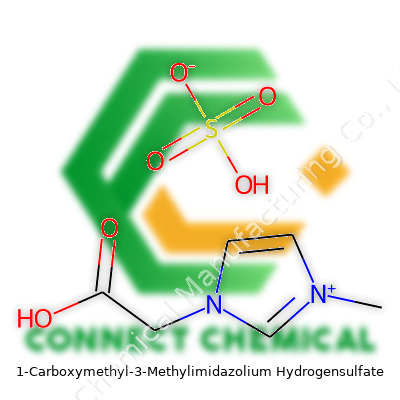
1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate: A Practical Perspective
Historical Development
The story of 1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate stretches back to those early years when chemists got curious about greener solvents and ionic liquids. For many decades, researchers relied on classic reagents that worked, but brought stubborn issues with toxicity and waste. Somewhere around the early 2000s, the field of room-temperature ionic liquids blew up, spurred by the search for alternatives to hazardous solvents. Chemists found that coupling imidazole rings with sulfate groups creates robust ionic liquids, opening doors to tunable physical properties and improved biodegradability. The carboxymethylation of the imidazolium backbone married solubility with reactivity, leading to compounds like 1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate, which quickly earned attention for unique performance in both academic and industrial settings.
Product Overview
This chemical—often shortened in conversation to CMMIm HSO4—falls in the category of functionalized ionic liquids. Its structure features a methyl group and a carboxymethyl arm on the imidazole ring, counterbalanced by hydrogensulfate. The pairing results in a clear, viscous liquid that resists volatility but delivers surprising reactivity. Lab professionals value it for the way it streamlines synthetic transformations, particularly acid-catalyzed ones, while keeping workplace hazards low. Through years on the bench, it’s gained a reputation for being forgiving—less corrosive than mineral acids and much easier to clean up.
Physical & Chemical Properties
Anyone who's handled CMMIm HSO4 notices straight away that it pours with the smooth, thick feel of glycerol, though its smell reveals a sulfur undertone. Its melting point sits well below room temperature, and it refuses to evaporate or burn easily, which helps keep it in place during reactions. Water loves to mix with it, but it stays mostly separate from many organic solvents. In my own experience, adding it to aqueous solutions gives a marked drop in pH—almost like adding a strong acid but without the wild exotherm or sharp fumes. Chemically, the compound refuses to join redox processes under gentle conditions, but its hydrogensulfate moiety passes along acidity with confidence, so it gets relied on in catalysis. Its ionic nature makes it a solid conductor, useful in electrochemical setups that need stable, non-volatile salts.
Technical Specifications & Labeling
Suppliers typically market CMMIm HSO4 with purity levels upwards of 95%, confirmed by NMR and elemental analysis. Color ranges from water-white to faint yellow, with transparency as a useful quick check for cleanliness. Standard product labels flag the molecular formula (C7H11N2O5S) and a molecular weight near 250 g/mol. Labels also feature safety codes that stress handling with gloves and eye protection owing to its acidic nature. Any shipment worth using arrives in sealed, chemically resistant bottles or drums with a clear lot number for batch tracking—an accountability step that's proven key for reproducibility in research settings.
Preparation Method
Most published syntheses begin with N-methylimidazole, a staple that's cheap and readily available. The process usually starts by reacting this imidazole derivative with chloroacetic acid under basic conditions to slot on the carboxymethyl group. Next, anion exchange converts the chloride form to hydrogensulfate through treatment with sulfuric acid—an age-old trick that’s been refined in countless university teaching labs. Careful washing and vacuum drying finish the job. From batch experience, even minor shortcuts in the purification step often leave persistent starting material, underlining why each workup gets methodical attention. Waste from this process mostly falls into aqueous, biodegradable categories, a relief compared to older acid-catalyst preparations that left behind heavy-metal salts.
Chemical Reactions & Modifications
Through the past decade, researchers have leaned on CMMIm HSO4 for its acid-catalyzed power paired with remarkable selectivity. Its applications cut across esterification, transesterification, Fischer-type reactions, and a growing list of ring-opening polymerizations. In experiments, it sometimes outshines classic mineral acids by avoiding overreaction and excessive byproduct formation. The carboxymethyl side chain sits ready for further modification, letting chemists graft in other functionalities for specific jobs—like ionic liquid polymers or task-specific solvents. Anyone who's tried to tweak its backbone recalls the solubility headaches from more complex derivatives, but the base molecule stays far more manageable.
Synonyms & Product Names
Chemical catalogs call it 1-carboxymethyl-3-methylimidazolium hydrogen sulfate, but it shows up under commercial abbreviations like CMMIm HSO4, or even as CMIMH for those who want to keep file names tidy. Some suppliers offer it as a member of "functionalized imidazolium ionic liquids," but the IUPAC name remains the safest reference in regulatory paperwork.
Safety & Operational Standards
This chemical demands basic precautions thanks to its acidity, though it doesn’t bring the dangers of concentrated sulfuric acid or HCl. Running new reactions means checking SDS sheets and watching out for splashes—skin contact causes mild irritation, while inhaling vapor could sting the nose but rarely occurs thanks to its low volatility. Spill cleanup feels reassuringly straightforward: plain water, lots of it, followed by mild base neutralization for stubborn spots. Labs treat waste runs as for mild acids, skipping the need for hazmat disposal teams. I’ve found that students pick up confidence with this type of reagent much quicker than with older acid catalysts, which encourages broad use in teaching settings.
Application Area
No singular application defines CMMIm HSO4; instead, the chemical has found work across biodiesel production, organic synthesis, biomass conversion, and some niche roles in analytical chemistry. Biodiesel plants value its role in transesterification of fats to methyl esters, as it offers solid yields with simpler cleanup. Synthetic chemists working on polymeric materials appreciate its stability in acidic regimes. I’ve seen research groups tackle challenging dehydration and cyclization reactions without resorting to classic mineral acids, improving yields and cutting hazardous exposure. Analytical professionals use it for some ion-exchange chromatography tasks, taking advantage of its ionic strength and pH buffering capacity.
Research & Development
The pace of R&D surrounding CMMIm HSO4 keeps gathering steam. At every major chemistry conference, talks surface on green catalysis using functionalized ionic liquids. Labs keep publishing case studies showing reduced environmental impacts and new chemical reactivities. In hands-on work, the focus turns to task-specific tailoring—altering the carboxymethyl group or ionic partner to enhance selectivity, or boost performance in particular reaction schemes. Startup companies chase new formulations that deliver cheaper, safer catalysts for both large-scale plants and boutique chemical syntheses, suggesting the waters are still deep for future discovery.
Toxicity Research
Toxicological studies on the base imidazolium ionic liquids generally suggest low acute toxicity to humans, with effects mostly limited to skin and eye irritation. Chronic exposure data piles up more slowly, and ongoing environmental assessments still urge caution, as imidazolium compounds linger in aquatic systems; this keeps wastewater disposal reviewed by environmental officers. The hydrogensulfate anion dilutes some safety risk, though the acid character means aquatic toxicity assessments measure against a backdrop of pH disturbance rather than unique molecular toxicity. So far, regulatory agencies haven’t flagged significant restrictions, but a prudent chemist keeps up with fresh findings, especially where green chemistry and closed-loop systems come into play.
Future Prospects
With growing pressure to dump legacy solvents and mineral acids in favor of greener systems, CMMIm HSO4 sits poised for wider adoption. Its growing track record in catalysis, efficiency, and safer handling should only strengthen as industries transition to sustainable operations. Breakthroughs seem likely as material scientists and synthetic organic chemists customize its structure for specialty applications—such as tailored polymer electrolytes, recovery solvents, or biomass conversion technologies. My own view is that the future belongs to reagents which answer real-world pressures for safety, recyclability, and scalable performance. People working with this ionic liquid would do well to keep an eye on advances in process integration and eco-toxicological assessment, ensuring each step forward comes with fewer trade-offs and a lighter environmental footprint.
A Unique Compound With Real-World Impact
1-Carboxymethyl-3-methylimidazolium hydrogensulfate sounds like the kind of chemical name only a specialist could care about. People who spend time in chemistry labs or work on sustainable technology will tell you there’s a good reason it gets attention. This compound belongs to a family known as ionic liquids—substances that act like salts but melt at much lower temperatures. Because they’re liquids at room temperature, they tend to solve a long-time problem in chemistry: finding alternatives to harsh, polluting organic solvents.
Green Chemistry Turns to Ionic Liquids
Every day, industries and research labs want to clean up their act. Solvents like toluene or chloroform do their job, but they punch above their weight in terms of pollution and health hazards. Based on my own experience in the lab, whenever someone mentions ionic liquids, most folks look for compounds that combine effectiveness with easy handling. That’s where 1-carboxymethyl-3-methylimidazolium hydrogensulfate lines up—it serves as a safer choice for dissolving other chemicals and getting reactions done.
Catalyst for Cleaner Industrial Reactions
This compound steps up beyond the typical solvent role. It’s proving essential for catalyzing processes that normally need strong acids—think making biodiesel or breaking down cellulose from plant waste. You can run these reactions without spewing corrosive fumes or worrying about complicated disposal problems. Researchers highlight lower toxicity and the promise of using this liquid more than once, making it attractive to industries that don’t want heavy cleanup costs.
Bringing Down the Barriers in Extraction and Separation
Many chemical plants wrestle with separating one ingredient from another, especially in pharmaceutical production. Traditional solvents work, but they often strip out more than you want and leave behind residues. In my graduate studies, I saw teams turning to ionic liquids like this one for extracting metals, dyes, and active ingredients from solutions. The selectivity comes from the structure itself—this imidazolium-based material binds well with certain ions or organic molecules, letting chemists target exactly what they need.
Supporting Sustainable Solutions
Global demand is rising for greener ways to process materials—whether in recycling, water purification, or specialty manufacturing. 1-Carboxymethyl-3-methylimidazolium hydrogensulfate answers the call by offering low volatility. That’s important because it keeps chemical losses and emissions from rising during use. Scientists love having a material that doesn’t evaporate easily or ignite. Risks tied to storage, spills, or exposure to heat drop considerably. The fact that this compound can often be recycled right back into the next round of production turns it from a one-off solution into a real workhorse for sustainable chemistry.
Getting Past the Limits
No chemical compound turns every process green overnight. Costs, access, and the nuts and bolts of scaling up from lab bench to factory floor present real challenges. Even so, as research digs into ways to make ionic liquids easier to produce and dispose of safely, the appeal keeps growing. Chemical supply companies now offer this kind of ionic liquid in larger and purer quantities. Collaboration—between industry, regulators, and academic groups—helps address gaps in safety guidelines and recycling practice. The path forward relies on sharing results, improving safety data, and keeping an eye toward closed-loop processes that cut down on waste and cost.
Getting to the Heart of the Matter
Every strange-sounding chemical name tells a full story. In the lab, 1-Carboxymethyl-3-methylimidazolium hydrogensulfate isn’t just a tongue-twister. The challenge starts with translating that mouthful into something that anyone can actually use—its chemical formula: C7H12N2O5S.
Why Chemical Formulas Matter
A chemical formula gives chemists a kind of shorthand, a street map for molecules. One glance, and a trained eye spots what’s hooked together and where. With C7H12N2O5S, you’re looking at a combo of carbon, hydrogen, nitrogen, oxygen, and sulfur, strung together into a structure that packs purpose. Whether mixing it up in graduate school to study ionic liquids or reading chemical patents, using formulas correctly saves time, money, and even keeps people safe.
1-Carboxymethyl-3-methylimidazolium hydrogensulfate stands out as a type of ionic liquid—those are salts that stay liquid at fairly low temperatures. They pop up in surprising places: greener solvents for cleaning up chemical reactions, maybe in battery research, or as catalysts for breaking down biomass. The precise formula tells anyone handling the stuff exactly what to expect and how to store or dispose of it.
Learning Through Experience
Sometimes as a student, I’d stare at the label on a bottle and feel like my brain ran out of gas. Experience changes the picture. Once you get the hang of reading these formulas—knowing C7 means seven carbons, H12 is twelve hydrogens, and so on—it starts to take shape. I started caring a lot more about accuracy when a miswritten formula led to wasting hours mixing the wrong reactants. That kind of mistake drives home the real meaning hidden in a simple string of letters and numbers.
Chemical formulas are more than abstract information from textbooks. During research, one slip in a formula can cost thousands in wasted materials or ruin a project’s safety record. The hydrogensulfate part in this case signals acid properties. The wrong formula means mixing something hazardous by mistake, putting health on the line, not just grades.
Problems and Practical Solutions
People make mistakes, especially newcomers. The maze of chemical names and formulas easily overwhelms learners. Clear labeling, double-checks, and up-to-date training help cut down on mistakes. Labs ought to back up written formulas with structural drawings, so folks start to connect the symbols with real compounds. Digital databases like PubChem or ChemSpider streamline this process by cross-referencing names, structures, and formulas, adding a layer of security. Peer review before running an experiment or peer check of your notes can catch errors strong enough to cause a spill or worse.
Chemistry works best with a little humility and a lot of respect for detail. Down the road, as new chemicals with even stranger names pop up for cleaner industry or smarter batteries, being able to translate those fancy names back to a basic formula will matter more than ever. The formula C7H12N2O5S—and getting it right—may sound like a small thing. Anyone who’s botched an experiment because of one wrong letter knows just how big an impact those symbols have.
Understanding the Substance
1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate popped up in research labs as an ionic liquid, often promoted for its supposed green appeal. On paper, it replaces harsher solvents and gets used in chemical synthesis, catalysis, and some electrochemical processes. Some industry pros say ionic liquids shift the game by lowering volatility and supposedly cutting emissions.
Health Hazards: Is the Concern Real?
Reality check—just because a compound breaks away from tradition doesn’t guarantee it’s harmless. For many ionic liquids, data on long-term health effects stays thin. This compound, like others in its family, brings a mix of positives and unknowns. Oral, skin, or inhalation exposure hasn’t been researched enough. What’s on record: lab studies on related chemicals sometimes show toxicity to aquatic life and moderate irritation to human tissues.
Personal experience as a lab technician and in regulatory safety assessments has taught me that the mere absence of a pungent odor or volatile fumes lulls folks into a false sense of security. Ionic liquids can persist in the environment longer than expected, and some break down into hazardous byproducts. One published study (2010, Green Chemistry) tracked several imidazolium-based ionic liquids, pointing out low biodegradability and evidence they can damage aquatic organisms at low thresholds.
Environmental Effects
This chemical, dumped or spilled, can stick around. Waste teams have told me how treatments for typical lab solvents sometimes fail on these newer substances. Wastewater plants struggle to filter out ionic liquids since they resist breakdown. That creates trouble downstream: residues from one mishap may find their way into rivers or soil. Once there, long half-lives mean risks multiply, especially if organisms start accumulating the stuff.
Digging through safety data sheets and published research, I also found that commercial suppliers still call for gloves, goggles, and good ventilation during handling. That’s not just lawyer-speak—it signals the unknowns lurking. Some countries, like Germany, have started evaluating these “greener” solvents for stricter regulation after toxicity reports emerged.
What Should Professionals Do?
Safety professionals can’t shrug this off as just another lab chemical. Expecting a “green” label to cover all bases would be a mistake. Teams using 1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate—whether in pilot plants or R&D—do better with a risk management approach. Minimize direct contact, and keep work in well-ventilated hoods or closed systems. Emergency response plans need updating for substances that don’t fit the old mold.
Companies and labs ought to push for transparency, sharing any incident data. Supporting open access to new toxicological studies can bridge gaps, pushing regulators to lay down evidence-based guidelines instead of just reacting. Institutions like the EPA and European Chemicals Agency already track these chemicals; stronger reporting helps them update rules in real-time.
Finding a Path Forward
It’s easy to hype new chemistry, but real trust comes from surfacing both pros and cons. A culture of safety can grow when departments test new materials on small scales first, keep protective measures high, and partner with waste managers on better disposal. Pressing for green chemistry means keeping an eye on hidden hazards—not just celebrating what’s shiny and new. Anyone working with 1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate owes it to colleagues, the environment, and the next generation of chemists to treat the unknowns with respect, not shortcuts.
Understanding the Substance
Working in a laboratory setting teaches a thing or two about the quirks each chemical brings along. 1-Carboxymethyl-3-methylimidazolium hydrogensulfate, an ionic liquid, stands out for its unique physical and chemical traits. These can offer impressive advantages for green chemistry, but they bring real-world headaches if ignored.
Temperature and Moisture Matter
This compound doesn’t play well with humidity. I remember opening a bottle after a few careless weeks on a standard shelf. Clumpy. Hard to handle. Moisture can mess with its purity, and that alone drives home the lesson: keep it tightly sealed and stash it somewhere dry. A desiccator or a humidity-controlled cabinet avoids all that trouble. The right storage keeps the material flowing, prevents unintentional reactions, and protects all those expensive reagents in the process.
Light and Air Exposure
I’ve seen researchers underestimate the power of environmental exposure. For many ionic liquids, a bit of sunlight or oxygen is enough to trigger degradation or discoloration. This one’s no exception, especially over longer stretches. That’s why an amber-colored, airtight glass bottle is usually the way to go. It keeps the contents clean and extends shelf life without any fancy tricks.
Safety Gets Real in a Busy Lab
Packing chemicals away isn’t just a box-checking exercise. There’s a real risk if the wrong step is skipped, especially here. 1-Carboxymethyl-3-methylimidazolium hydrogensulfate packs acidity, and if it spills, you want to minimize damage. I’ve knocked over bottles before—no fun at all. Secondary containment trays are a cheap insurance policy. Spill kits belong nearby, so any mess gets dealt with fast, before it turns into a bigger problem. Personal protective equipment—gloves and lab coats—make cleanup less of a gamble. Every incident I’ve witnessed pushes these lessons home further.
Labeling Isn’t Optional
Busy benches and shelves get crowded, and mistakes creep in faster than anyone would like. Mistaken identity doesn’t just ruin experiments, it poses serious danger if incompatible chemicals mix. So, every bottle has a label with the compound’s full name, concentration, date of receipt, and hazard info. Getting it wrong creates confusion—it only takes one misplaced drop to cause chaos. Keeping things straightforward cuts down on these headaches.
Disposal and Longevity
No one wants a buildup of half-used, aging chemicals. Unused material eventually breaks down, which messes with experimental data and increases risk. Routine inventory checks help keep only what’s needed on hand. Outdated stock goes to chemical waste collection, and that keeps both results and coworkers safer. Regular inventory checks shouldn’t be optional—they are every bit as crucial as setting up a reaction right.
Better Storage Saves Time and Money
It’s easy to look past good habits, but well-organized storage delivers fewer surprises and lower costs. When the right containers, controlled environments, and careful labeling line the shelves, results come out cleaner and fewer accidents make headlines. These efforts serve not just those at the bench but everyone up and down the line, from the cleaning crew to executive management. The key is simple: treat the chemical with respect and it returns the favor over and over again.
Checking Purity: More Than Just A Number
Purity for chemicals like 1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate shapes the kind of results researchers and manufacturers get. Labs looking for consistent ionic liquid performance usually run into purity grades in the 97% to 99% range. This degree of purity comes straight out of conversations with chemists and suppliers who measure success by how little contamination sneaks in. Labs tend to favor lots where impurities stay just under control because even tiny additives mess with key reactions, particularly in catalytic and electrochemical research.
Why High Purity Makes a Difference
Ionic liquids find their way into reaction screening, solvent systems, specialty catalysis, and even battery electrolytes. I’ve seen projects completely derailed because a compound came in just a percentage point too low in purity. Contaminants like moisture or trace metals disrupt reactions or shorten the lifespan of an ionic liquid. If you’re pushing toward results you can trust, 99% is not an arbitrary marketing stunt. Instead, it’s a practical necessity for reproducible data and regulatory audits.
Testing Methods: How Purity Gets Verified
Labs and producers don’t only rely on trust; there’s always a report or certificate backing up these numbers. Look for suppliers who provide NMR, HPLC, or elemental analysis data, and, where necessary, are open about residual solvents or trace cationic or anionic content. Some vendors can deliver a dry, colorless ionic liquid that passes Karl Fischer for low water and ICP-MS for no heavy metals. If a chemical comes without this paperwork, expect problems down the road. I’ve spoken with colleagues who got stuck re-working months of data simply because a cheap sample turned out only 95% pure, missing those extra checks.
Market Trends and Buyer Tips
Over the past few years, demand for advanced ionic liquids has nudged suppliers to step up their game. High-throughput labs, green chemistry initiatives, and next-gen batteries all require higher grades to give meaningful, scalable results. Trusted suppliers usually offer both analytical data and supply chain transparency, which helps weed out dubious grades and gray-market material. Yet the temptation always pops up when a lower-cost option appears online. My advice—don’t go for the cheapest line item unless there’s a clear purity statement and independent verification. Reach out to vendors ready to discuss quality control, not just their price list.
Fixing Sourcing and Purity Challenges
Labs and industrial buyers can dodge many headaches by building relationships with reliable distributors and regularly checking certificates. Bulk buyers sometimes negotiate for custom batch purification, putting pressure on suppliers to filter, dry, and analyze the product before shipping. I recommend keeping a small sample city—one portion sealed off as a backup for reanalysis long after the shipment lands. Getting too comfortable with standard grades leads to surprises after scale-up or during regulatory checks.
The Takeaway
At the end of the day, chemical purity reflects the health of your science or process. Ionic liquids like 1-Carboxymethyl-3-Methylimidazolium Hydrogensulfate arrive at 97% to 99% purity from major sources, and that window sets the standard for technical and research reliability. Personal experience and industry best practices both point to the same path: check the paperwork, inspect the source, and never rely on guesswork.