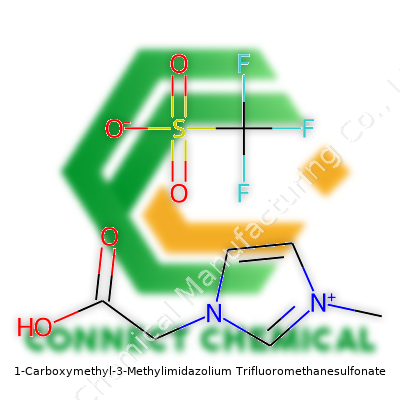
1-Carboxymethyl-3-Methylimidazolium Trifluoromethanesulfonate: A Practical Look
Historical Development
Back in the late 20th century, chemists dug deep into new classes of ionic liquids that kept showing promise in “green chemistry.” Among them, 1-carboxymethyl-3-methylimidazolium trifluoromethanesulfonate sparked real curiosity, quickly winning attention from both academic and industrial researchers. Originally, scientists pushed for alternatives to volatile organic solvents and traditional phase-transfer agents. The early research focused on stable, non-flammable liquids that could replace hazardous solvents in labs and processing facilities. As regulatory agencies pressed industry to clean up emissions, practical need for these new ionic liquids stoked rapid advancement. Over the next two decades, improvements flourished, shaped by improved analytical tools like NMR and mass spectrometry—tools that let scientists tinker with these compounds at the atomic level and get a clear picture of how they behaved in real-world applications.
Product Overview
Looking at 1-carboxymethyl-3-methylimidazolium trifluoromethanesulfonate, you’ve got a salt formed from a methylimidazole core—an organic ring that brings stability—and a carboxymethyl side chain for extra solubility in polar environments. Toss in that hefty trifluoromethanesulfonate anion, and you get a compound with real promise for a greener chemistry bench, often used where traditional solvents pose environmental or safety problems. With this particular structure, chemists rely on its ionic nature to dissolve both organic and inorganic compounds, letting reactions proceed cleanly without much fuss about hazardous waste. Industrial producers deliver this product as a colorless to pale yellow viscous liquid or as an easily handled solid, always packaged in airtight containers to protect from moisture and environmental contaminants.
Physical & Chemical Properties
1-carboxymethyl-3-methylimidazolium trifluoromethanesulfonate stands out with a melting point low enough for most practical lab uses and a remarkable thermal stability—up to about 250°C in most cases. It resists oxidation, and its broad electrochemical window keeps it useful for a range of applications, from catalysis to electrochemistry. High ionic conductivity makes it an appealing choice in electronics and batteries, and it holds up under strong acidic or basic conditions better than most organic solvents. Hydrophilicity, aided by the carboxymethyl group, allows it to blend well with water, while its solvation power stretches across polar organic molecules and some inorganic salts. Most labs measure density at around 1.45 g/cm³, and viscosity settles in the moderate range, which helps accelerate mass transfer in reactions or extraction processes.
Technical Specifications & Labeling
Producers provide detailed labeling for this chemical, tracking lot numbers and specific purity, commonly exceeding 99% on a dry basis—a must for research and pharmaceutical work. Common labels show the full systematic name along with alternative product names to avoid confusion during ordering or regulatory checks. Shipment always includes hazard data, directions for safe storage, and recommendations for handling personal protective equipment (PPE). Laboratories may ask for certificates of analysis, confirming compliance with GMP or ISO standards where applicable. Chemists pay special attention to moisture content and trace metal impurities, as just a few parts per million can throw off delicate syntheses or cause problems in catalysis.
Preparation Method
Making this ionic liquid often starts with methylimidazole as the base. The synthesis typically kicks off with N-methylation, followed by reaction with chloroacetic acid in an alkaline medium to graft the carboxymethyl side chain onto the nitrogen atom. Next, as the trifluoromethanesulfonate salt forms through either direct acid-base neutralization or via ion exchange resin, purification begins—sometimes with repeated recrystallization, other times with rotary evaporation and drying under vacuum to control residual solvent levels. Throughout, pure water and reagent-grade solvents keep contamination down, and each step involves careful pH and temperature adjustment to secure a consistent product every batch.
Chemical Reactions & Modifications
At the bench, 1-carboxymethyl-3-methylimidazolium trifluoromethanesulfonate serves well as a starting point for more intricate ionic liquids—one reason it’s found in custom formulation work. Chemists attach various functional groups to the imidazolium ring or the carboxymethyl arm, making derivatives that match specific reactivity or solubility. The trifluoromethanesulfonate counterion swaps out in metathesis reactions, opening up more room to tailor properties like coordination strength or hydrophobicity. This ionic liquid dissolves many organic and inorganic substrates, allowing direct participation in reactions, especially as both solvent and catalyst in cycloadditions, SN2-type displacements, or selective oxidations. Researchers report using it to mediate phase-transfer or extraction processes, especially for metal complexes or pharmaceutical intermediates.
Synonyms & Product Names
Other names crop up on supplier lists just to keep things clear—look for “1-Carboxymethyl-3-methylimidazolium triflate,” “CMIM OTf,” or “1-CM-3-MIM trifluoromethanesulfonate.” Some catalogs shorten to acronyms like “CMMIM OTf.” Trade names come up, though rarely, since most usage centers on direct, scientific procurement rather than branded, commercial products. Labs sometimes abbreviate internally for ease of record-keeping.
Safety & Operational Standards
Like all high-purity ionic liquids, this compound benefits from strict hazard protocols. While its low vapor pressure and thermal resistance reduce the chance of fire or major volatilization, handling guidelines call for gloves, goggles, and a clean bench or fume hood setup. The carboxymethyl group and the strong triflate anion block much direct irritation, but chemists still avoid direct skin or eye contact. Spills call for inert absorbents, and waste management teams separate spent liquid for specialty chemical disposal, following local hazardous waste rules. Producers supply safety data sheets that map out emergency procedures for accidental exposure or fire. In practice, researchers emphasize airtight storage to keep out moisture that could affect reactivity or shelf life.
Application Area
Researchers and manufacturers alike appreciate this salt’s versatility. It steps in as a reaction medium in organometallic syntheses, especially for couplings, cycloadditions, and oxidations. Battery tech companies see its ionic conductivity as an asset for electrolytes in advanced lithium battery prototypes. Extraction work in pharmaceutical processing draws on its solubility profile, while biocatalysis applications benefit from its ability to stabilize proteins and enzymes under tough conditions. Environmental labs use it to extract and analyze heavy metals in water, since the carboxymethyl group can chelate a variety of metal ions. Its compatibility with water and resistance to breakdown under acidic or basic stress point to high-value applications in the production of fine chemicals, nutraceuticals, and specialty polymers.
Research & Development
Development cycles in academia and industry keep pushing the envelope for ionic liquids like this one. Researchers hunt for ways to further drop costs by using recyclable catalysts or new synthetic methods. Labs use analytics to optimize parameters—balancing conductivity, viscosity, and environmental safety—by swapping structural groups or tweaking counterions. Companies diving into green chemistry tout its reduced toxicity and waste product compared to classic solvents, producing less air and water pollution in pilot studies. In pharma, exploratory projects target custom-formulated ionic liquids for use as drug delivery vehicles and stabilizers for sensitive proteins. Funding comes from public and private sources, giving a real boost to cross-disciplinary work between chemistry, engineering, and material science.
Toxicity Research
Routine assessments suggest this compound has significantly reduced volatility and acute toxicity than many traditional organic solvents, yet no one treats it as harmless. Standard cell studies and animal tests show some cytotoxic effect at higher concentrations or with chronic exposure, especially in aquatic environments. The triflate anion, known for its stability, proves tough for most microorganisms to break down, raising potential persistence concerns. Regulatory reviews in North America, Europe, and Asia drive manufacturers to conduct comprehensive ecotoxicity and biodegradation studies. Regular monitoring tracks disposal patterns to avoid build-up in groundwater or industrial effluents. Industry insiders watch upcoming toxicity reports closely, since one bad result could disrupt many planned uses in food or pharma.
Future Prospects
Future trends keep turning toward custom ionic liquids tuned to solve tough chemistry problems, and this triflate salt holds a strong spot on that list. As manufacturing groups lock in automation, process control, and improved recycling for ionic liquids, producers expect operational costs and waste to fall. Researchers expect adoption into broader industries—energy, water treatment, and even electronics—if they can prove up safety and low environmental impact. New computational tools help speed up the discovery cycle, letting labs pinpoint application-specific ionic liquids in silico before ever running a reaction on the bench. The next few years may see this compound form the backbone of designer solvents and green electrolytes. Weighing all this, the real test lies in field-performed risk assessments and life cycle studies—those results will shape how far and fast the technology spreads.
The Appeal of Ionic Liquids
In labs around the world, researchers turn to chemicals that get tricky jobs done cleanly. 1-Carboxymethyl-3-methylimidazolium trifluoromethanesulfonate is one of those unsung workhorses known as an ionic liquid. A lot of chatter happens about them because these salts stay liquid even at room temperature. This property gives them a broad playground in chemistry, making tasks that usually push us to the edge of frustration much easier.
A Green Push in Chemical Processes
Many chemical reactions spit out waste and need troublesome solvents. Scientists have started to swap in ionic liquids for old-school solvents that bring heftier environmental baggage. I’ve seen 1-carboxymethyl-3-methylimidazolium triflate earn praise for helping clean up reactions. The stuff barely evaporates, so it doesn’t send toxic fumes flying around the room. The reduced volatility means safer labs, cleaner air, and less headache for folks working long shifts.
Take organic synthesis, for example. Here, precision is non-negotiable, and yield matters. This ionic liquid can act both as a solvent and sometimes nudge the reaction along as a catalyst. It handles stubborn molecules and supports the transport of ions, helping reactions finish with fewer impurities. Chemists in pharmaceutical and fine chemicals manufacturing turn to substances like this to pump up production and lower clean-up costs.
Facilitating Electrochemistry and Energy Storage
Ionic liquids get a lot of attention in batteries, supercapacitors, and similar energy tech. Standard electrolytes often break down fast, especially under high heat or after long use. 1-Carboxymethyl-3-methylimidazolium trifluoromethanesulfonate resists decomposition, and its stability means safer and longer-lasting battery cells. I keep up with battery research, and over and over, I see ionic liquids used to tackle flammability and leakage—two headaches that short-circuit innovation elsewhere.
Researchers at universities and private companies try ionic liquids to tune the properties of electrolytes in lithium-ion and other battery types. The goal is a battery that holds more juice, works at wider temperatures, and doesn’t catch fire if something goes wrong. These aren’t just small differences. Better and safer batteries open the door for more reliable electric vehicles and greener energy grids.
Taming Tough Pollutants
Extracting and purifying compounds from stubborn mixtures usually needs harsher chemicals or loads of water. Ionic liquids like this one offer a gentle but powerful way to pull useful stuff from waste. They separate metals and organics more efficiently than many tried-and-true solvents. For water treatment facilities, mining, and recycling, this can mean capturing valuable elements with less impact.
I know wastewater plants where engineers constantly look for ways to trap heavy metals that slip through conventional filters. Ionic liquids bring a toolkit for selective extraction, helping snag pollutants before they reach rivers and farms.
Learning from the Evidence
None of these breakthroughs matter if they don’t carry evidence behind them. Studies in journals like Green Chemistry and Journal of Power Sources have detailed how this specific ionic liquid shrinks toxic solvent use and, in batteries, delivers stable electrical performance over time. Data stays king as companies and regulators weigh the cost and benefits of greener options.
labs still face challenges, like recycling ionic liquids and keeping costs reasonable. Scaling up means paying close attention to sourcing and managing waste. Some forward-thinking chemists explore ways to recover and reuse these liquids instead of tossing them out. It takes creativity and steady hands, but that's where progress often shows up.
The Basics Matter More Than You Think
Every product tells a story. From fresh groceries to pharmaceuticals, it doesn’t take long for careless storage to ruin the ending. In my years working around food service and helping family-run shops, storage rules rarely felt optional. A crate of apples at room temperature on a summer day turns soft and bruised by next morning. A box of antibiotics left next to a sunny window raises doubts about whether anyone can trust what’s inside. At the core, storage conditions set the stage for everything that comes after, including safety, effectiveness, and satisfaction.
Temperature Isn’t Just a Number
I’ve watched small mistakes snowball just because “the room felt cool enough.” Temperature guides might look strict, but research consistently shows spoilage, bacterial growth, and loss of potency climb fast as temperatures edge up. The U.S. Food and Drug Administration and the World Health Organization make clear that storing perishable or sensitive items in the right temperature band greatly extends shelf life and cuts risk. Some products demand refrigeration between 2 and 8°C, others need freezing, while dry goods prefer cool, dark cupboards. Ignore the recommended zone and you might lose not just taste or appearance, but nutritional value — or, in some cases, safety.
Humidity and Light Make or Break Integrity
It’s easy to shrug off excess humidity or a bit of light. As I learned watching a bakery spoil its own flour, moisture sneaks into open bags and brings out the worst in grains, herbs, or powders, from clumping to mold. Medicines and supplements also react poorly to damp settings; the U.S. Pharmacopeia highlights this, warning that high humidity can trigger chemical changes. Transparent packaging in direct sunlight fades colors, robs vitamins of potency, and melts chocolates into a sticky mess. Even a glass of water tastes different if left under the light too long. Decent ventilation and opaque storage fix a lot — small actions with outsized impact.
The Role of Cleanliness and Labeling
It’s easy to toss everything on a shelf and hope for the best, but that shortcut causes problems. Cross-contamination isn’t some rare event — it pops up often when open containers stack near one another or when surfaces stay grimy. Keeping storage areas clean reduces the risk of bacteria, pests, and foreign residue. Accurate labeling matters too. Mislabeled products end up used past their best date or mixed up with allergens, causing costly or dangerous mistakes. Labeling, along with a clear first-in, first-out rule, lets everyone see exactly what’s in stock and what should be used up next. Organized systems translate into fewer errors and less waste.
Simple Steps, Big Results
People often search for fancy equipment or high-tech solutions, but the basic rules carry more weight than the next new gadget. Businesses thrive when employees understand why storage rules exist. Consumer trust follows when brands clearly share their handling processes. Whether it’s rotating stock, checking fridge logs, or tracking changes in humidity, these routine habits set strong foundations. For folks running a kitchen or a warehouse, training and regular checks help spot trouble before products end up lost or unsafe. Sharing storage guidelines — clear, easy to follow — gives consumers a path to confidence as well. Everyone from producer to shelf-stocker plays a part.
Small Changes, Less Waste
By paying close attention to storage, countless pounds of food, medicine, and resources avoid landfill. The United Nations Food and Agriculture Organization estimates one-third of food produced gets wasted, much of it because of poor storage. Simple changes — better airflow, more consistent temperature, informed staff — can make a real dent in those numbers. It’s not about chasing perfection, just getting the basics right, every day, for every product on every shelf.
Calling Things Hazardous: It’s Rarely Black and White
I remember talking to a college chemistry teacher about a bottle of acetone sitting by the sink. I knew nail polish remover smelled strong, but reading “flammable” and “irritant” made it seem like touching it would spell disaster. That caution isn’t wrong—skin and eye contact matter, and open flames turn acetone into a fire hazard. The point is, fear alone doesn’t bring real safety. Information and practice do.
Folks use chemicals in daily life. Bleach brightens whites and keeps bathrooms clean. Farmers rely on pesticides that, applied carelessly, end up in drinking water. Whether a chemical worries people usually depends on the context. The same ammonia working in window sprays can damage lungs in a closed room, but it doesn’t bother folks diluted in a bucket.
How Experts Judge Danger
Groups like OSHA, the EPA, and Europe’s REACH program don’t just slap the word “hazardous” on a label for no reason. For anyone curious, their websites break down chemical safety into toxicology, exposure, and use. Let’s keep it real. Worker protections in a factory may not apply to folks at home, but every regulation on that bottle of oven cleaner means the regulator has looked at both long-term and short-term risks. Dose and duration count just as much as what spills out of the bottle.
Reading Labels vs. Going Down Rabbit Holes Online
People worried about chemical exposure sometimes fall down rabbit holes online. “Natural” rarely means safe. Take the example of arsenic in well water; the earth gives us plenty of that, and drinking it does real harm. Conversely, science-based evidence supports chlorine in small amounts for water disinfection, keeping entire cities healthy. Trustworthy sources matter. Labels on consumer products list hazards and first aid steps, drawn from long-term study and real cases. I’ve seen labels update after new research, especially for kids and pets.
What Matters in Everyday Life
Plenty of folks think common chemicals are fine—until something goes wrong. Mixing household cleaners, skipping gloves, or leaving bleach where kids can get it—these examples usually drive home how fast a “normal” substance becomes dangerous. It’s not about banning things outright. Education lowers accidents. When schools invest in safety goggles and consistent safety talks, accidents drop. My experience as a science volunteer in middle schools made it clear that real-life stories stick with students more than rules printed on the wall.
Who’s Most at Risk?
Kids, workers, and the elderly often deal with higher risks. Storage practices save lives: keeping toxic substances out of reach and away from food. For workers, policies about gloves, eyewash stations, and ventilation cut down on emergency room visits. In households, something as simple as using the fan and never mixing ammonia with bleach solves a lot of problems before they start.
Stopping Problems Before They Start
Good solutions don’t require a chemistry degree. Honest communication, clear labeling, and building habits around storage and clean-up matter most. Chemical manufacturers these days share more safety information than ever. Free apps scan labels and explain terms, though nothing replaces reading instructions all the way through. Changing how communities and workplaces talk about chemicals—from quiet warnings to open conversations—builds habits that last.
Hazards are real, but with respect, facts, and some common sense, chemicals do more good than harm.
The Real Story Behind Purity Specs
Product purity shapes almost every stage of production, whether we’re talking about pharmaceuticals, chemicals, or food ingredients. If you’ve ever spent time on the floor of a manufacturing plant, you know how much can hinge on what’s in the drum—or, more specifically, what's not in the drum. Purity isn't just a number on a certificate. It builds trust with customers, keeps regulatory headaches at bay, and makes sure that what you’re buying is exactly what you need.
Why Purity Matters Day-to-Day
Life moves fast for anyone who has to decide if a raw material is right for a product run. One phone call where a supplier says “this batch comes at 96% purity,” and you can feel the wheels turning. Will it work for our process? What’s the impurity? Could it ruin our batch or block us on compliance? Even one missed impurity risks damaging years of building a brand’s reputation.
According to data published in the Journal of Pharmaceutical Sciences, impurities—even in small amounts—can undermine performance, stability, and safety. One story that sticks with me is a quality audit where an excipient with a standard spec of 99% caused repeated batch failures at a plant. It took weeks to trace the cause to just 0.5% of a particular contaminant. Nobody wants to pull products or recall shipments, especially not for something that a tighter spec could have caught from the start.
The Fine Print—Specs Aren’t All Alike
All specs are not equal. A 99% purity written on paper doesn’t tell the whole story without details. Which impurities does that 1% include? Heavy metals, moisture content, residual solvents, and byproducts should come under the microscope. Let’s say you’re making food products—lead content needs to hit certain thresholds or you face legal trouble. In pharma, a small amount of the wrong organic compound can make the difference between a safe medicine and an unacceptable risk.
Certifications and independent lab testing go a long way here. Companies that invest in third-party verification help build confidence because you see not just the good news (high purity), but also the bad news (what’s left over, at what level). Regulators like the FDA ask for detailed analysis and proof, not just claims. The costs for poor specs can’t be ignored: product seizures, regulatory warnings, or worse, risk to public health.
Checking Specs Isn’t Just for Corporates
Even small outfits need to pay attention. In my early days at a startup, a single drum with a mislabeled purity spec nearly cost us our best customer. We had to spend precious cash sending it for confirmation testing. Since then, I always press for a full breakdown with every shipment. Seeing a full certificate of analysis with each delivery builds peace of mind.
Raising the Bar: How Do We Fix Gaps?
Tighter regulations will help, but supplier transparency goes further. A relationship built on honest sharing of test reports and rapid responses makes a difference. Real conversations about what minimum specs protect both sides. Investing in staff education also raises awareness; many issues come from not knowing what a particular impurity can do.
Ultimately, purity specifications aren’t just red tape. In every industry I’ve worked, checking them closely has saved money, time, and sometimes the company’s reputation. If both buyers and sellers put in the effort up front, everyone benefits—including the end customer.
Why Safe Practices Matter
Anyone stepping into the lab with 1-Carboxymethyl-3-Methylimidazolium Trifluoromethanesulfonate needs to respect the risks. A big part of this starts with knowing that the chemical contains both imidazolium and trifluoromethanesulfonate groups. Those who have worked with these components recognize their knack for causing irritation and, in some cases, long-term health risks if mishandled.
Clear memories from my own lab experience highlight how quickly things go south when chemicals like this get spilled or inhaled. One rushed day, a colleague neglected proper gloves when handling an ionic liquid. Not thinking through the possibility of skin absorption left him with an angry rash and sent us reviewing our protocols that afternoon.
Personal Safety Above All
Anyone weighing or transporting this compound needs to suit up. That includes chemical splash goggles, gloves made of nitrile or neoprene, and a lab coat. Airflow from a functioning fume hood keeps vapors away from lungs. Long sleeves and closed shoes aren’t negotiable; splashes can happen in a heartbeat.
Spills test people. I’ve seen panic set in, especially among new students. Good practice means reaching for absorbent materials and neutralizing agents. No one should sweep powder into the trash or rinse residue down the drain. Water alone can create more hazards, especially if the compound’s triflate end hydrolyzes under the wrong conditions.
Clean-up involves gathering the chemical and dirty materials, double bagging them, and labeling everything with the full name. Even at the end of the workday, surfaces where the compound touched require scrubbing with soap and water, discarding towels as hazardous waste.
Environmental Concerns and Regulatory Rules
The fluorinated part of trifluoromethanesulfonate stands out for environmental persistence. Nature doesn’t break these structures down easily. If they wash into soil or water, remediation demands more than time; it calls for effort, equipment, and funding. Years ago, regulators cracked down on perfluorinated chemicals for just these reasons. It pays to stay ahead of the curve instead of inviting inspection or liability.
No one wants to explain to a regulator how a mishap contaminated a drain. International standards, such as those from the EPA and REACH, list ionic liquids as chemicals of concern in some cases, especially the ones with persistent anions. In my own work, a surprise audit made me grateful for thorough documentation and a clear hazardous waste log.
Solutions for Disposal
No shortcut saves time or money in the end when it’s disposed of improperly. Approved waste services handle this kind of compound for a reason. They keep flammable and reactive compounds apart and bring the right knowledge for neutralizing hazardous fragments.
Every batch of waste needs clear labeling, including the chemical’s full name, concentrations, and date of disposal. I always send paperwork and Material Safety Data Sheets along with each container. Local policies might ask for more detail, especially if the compound’s production involves only small volumes. Lab managers who partner with licensed waste handlers build a track record of trust and avoid trouble.
Training and Culture
Every lab I’ve been part of relied on a culture of safety first. Nobody touches a new chemical without a talk on its risks and the right steps if something goes wrong. Regular drills keep the response quick. Solutions start with people knowing what’s at stake and being able to act fast, not just following a checklist.
Handling and disposal of 1-Carboxymethyl-3-Methylimidazolium Trifluoromethanesulfonate don’t need to slow science down. Respect for the risks, real world preparation, and committed partners make the work safer and smarter for everyone involved.