1-Carboxymethyl-Pyridinium Chloride: A Practical Look
Historical Development
Researchers in the mid-20th century began looking for pyridine-based chemicals that could carry functional groups with ease. 1-Carboxymethyl-pyridinium chloride first caught attention in academic circles during studies on organic ionic compounds and their behaviors. Chemists found practical uses for pyridinium salts in medicinal and material science. Later, industry started looking at these molecules for their versatile ionic properties, making them valuable in catalysis and pharmaceuticals. Over decades, the interest shifted from academic curiosity toward genuine commercial application, especially as technology demanded better, more stable intermediates for synthesis.
Product Overview
1-Carboxymethyl-pyridinium chloride presents itself as a versatile cationic compound. Specialists can appreciate its stability and water solubility. The chloride counterion gives it a neutralizing touch, and the carboxymethyl group opens doors to modifications. This reagent shows up in both analytical labs and pilot plants, playing a central role when researchers explore enzyme inhibition, molecular recognition, or electrochemical behavior. Because it’s not as mainstream as some reagents, buyers usually get it through specialist chemical suppliers that focus on research-grade compounds.
Physical & Chemical Properties
This compound typically appears as a fine, hygroscopic white powder or crystalline solid. 1-Carboxymethyl-pyridinium chloride dissolves well in water and polar solvents, owing much of its solubility to its charged pyridinium core. Melting points can hover around 190-220°C under normal lab settings. The molecule carries a permanent positive charge on its nitrogen, derived from the pyridinium group, which then binds tightly to the carboxymethyl side chain via nucleophilic substitution. Its ionic nature means it rarely serves as a neutral molecule in reaction sequences. It exhibits moderate thermal stability, handling standard drying and storage conditions, but strong oxidizers or bases can break it down. Keep it in dry, cool, airtight containers, since it pulls moisture from the air pretty quickly.
Technical Specifications & Labeling
Reputable sellers offer analytical and technical grade batches, each showing purity levels (usually 97% and up according to HPLC or NMR). Labels clearly state lot numbers, CAS number, molecular formula (C7H8ClNO2), and molecular weight (173.6 g/mol). Suppliers include Material Safety Data Sheets with hazard warnings and recommended storage. Well-packed glass bottles or HDPE jars protect against light and moisture. For transport, UN numbers or hazard symbols stem from national and international chemical guidelines, reflecting its mild irritant or corrosive potential. Users in regulated labs must log the reagent’s use due to compliance with chemical hygiene plans.
Preparation Method
The usual synthesis relies on nucleophilic substitution. Most chemists start with pyridine, reacting it with monochloroacetic acid under aqueous or alcoholic conditions. This forms the carboxymethyl-pyridinium ion, which then meets hydrochloric acid to introduce chloride as the counterion. Recrystallization from ethanol or acetone helps clean up the product and secure good yields. Some researchers prefer micellar or phase-transfer approaches to bump up rates or lower waste, depending on what the lab setups and budgets allow. It isn’t a compound that demands special tools; classic glassware, fume hood ventilation, and routine analytical checks get the job done.
Chemical Reactions & Modifications
1-Carboxymethyl-pyridinium chloride stands out as a ready substrate for modification. Its carboxylate function enables amide bond formation, coupling chemistry, and salt metathesis. That side chain invites reactions with alcohols, amines, or even peptides, broadening its use in bio-conjugation. The pyridinium ring tolerates methylation or other electrophilic substitution if conditions favor selectivity. Some research focuses on turning this base molecule into ionic liquids or functionalized supports. When heated with strong nucleophiles, ring opening or N-dealkylation can crop up, usually as an unwanted side effect, so control is crucial. Teams tweak pH, temperature, and co-solvents to coax the molecule into giving up its best features in multi-step syntheses.
Synonyms & Product Names
Besides the main name, chemical supply catalogs or technical papers might list N-(Carboxymethyl)pyridinium chloride, Pyridinium-1-yl-acetic acid chloride, or Pyridinium acetic acid chloride. Some vendors go for shorthand like CMP-Cl or Pyridinecarboxymethyl chloride. The same compound may pop up under different registry numbers, though reliable ones always cross-reference back to its accepted CAS identifier to prevent confusion.
Safety & Operational Standards
Handling this compound requires a common-sense approach learned from years in chemical labs. Gloves and goggles never leave the workbench. The main risk involves direct skin or eye contact, which might cause irritation or mild burns due to the chloride anion and the pyridinium cation’s reactivity. Fume hoods cut down on inhalation issues, especially for those with existing respiratory sensitivities. Spill response means lots of water, followed by neutralization. Storage involves sealed containers in low-humidity cabinets, out of reach from acidic or basic incompatibles. Waste gets bagged for licensed disposal or treated by neutralization in line with local environmental health and safety policies. Having witnessed many minor incidents with less-stringent compounds, clear SOPs and training create a safer work environment. Inspections and third-party audits help maintain standards.
Application Area
Teams in pharma, biochem, and material science all dip into stocks of 1-carboxymethyl-pyridinium chloride. Drug developers use it as an enzyme inhibitor scaffold or test its effect on biological membranes, since the pyridinium group often mimics choline-based neurotransmitters. Analytical chemists use its charged head group for ion chromatography, and electrochemists see it as a possible redox mediator or ionic conductor in prototype batteries. Synthesis requires intermediates with positive charges; this molecule answers that call, giving way to new catalysts, ionic liquids, and polymeric materials. Some environmental labs even tinker with it in water treatment studies, looking at how ionic additives can pull impurities from solution. Real-life applications depend on performance under pressure, and so far, feedback from those on the bench tells a positive story—no silver bullet, but a reliable tool in the chemical arsenal.
Research & Development
Academic labs focus on how structure tweaks shift properties. Some chase selective inhibitors for enzymes, others scan for better catalysts in tough conditions. The pyridinium core forms a backbone for ionic frameworks and tagged molecules for molecular recognition work. Funding agencies pay attention whenever data points toward easier, less wasteful syntheses or safer handling. Adding functional groups or pairing the chloride with alternative anions creates new niches. At conventions, researchers often mention how easy it is to modulate this molecule in combinatorial chemistry. The publication list grows slowly but steadily; real breakthroughs usually involve pairing 1-carboxymethyl-pyridinium chloride with advanced analytical tools or integrating it in eco-friendly products.
Toxicity Research
Most studies point to low acute toxicity at standard lab concentrations, but direct exposure tells a more nuanced story. Skin and eye irritation show up in lab safety logs. Chronic risk and organ-level impact remain less explored, though researchers keep an eye on pyridinium salts in general due to their structural similarity to biologically active compounds. Regulatory agencies usually lump in such quaternary ammonium or pyridinium derivatives for cumulative exposure limits. Animal testing at research institutions sometimes exposes minor enzyme inhibition, but results shift with slight chemical modifications. Proper reporting, MSDS compliance, and workplace controls make sure researchers limit their risk.
Future Prospects
1-Carboxymethyl-pyridinium chloride stands at the crossroads of routine chemistry and emerging tech. As synthetic biology, advanced materials, and green chemistry gain ground, demand for alterable, benign ionic reagents keeps rising. Battery engineers, drug developers, and wastewater specialists all keep an eye on new functionalized pyridinium derivatives for niche breakthroughs. Seeing firsthand the jump in research proposals and patents, practical chemists recognize the value of established, reliable compounds ready to be plugged into new systems. Further toxicity assessment and greener production methods remain front-line topics in workshops and industry meetups. Continuous refinement and expanded testing protocols will steer this compound’s journey forward, hopefully unlocking broader and safer pathways in tomorrow’s chemical landscape.
Digging Into Its Place in Modern Chemistry
Folks who have spent any time near a lab bench know some chemicals never make headlines but shape the way things work behind the scenes. 1-Carboxymethyl-Pyridinium Chloride walks quietly among them. While its name may trip up the tongue, no need to be intimidated. Chemists reach for this compound any time the goal pops up to activate carboxylic acids for coupling reactions—essential steps to create amides, peptides, and other molecules with real-world value.
Why This Compound Matters
Creating a peptide or fine-tuning a drug candidate often relies on precise activation steps. That’s where 1-Carboxymethyl-Pyridinium Chloride steps in. In medicinal chemistry, attaching molecules cleanly and efficiently can turn an idea on paper into a promising treatment. This compound helps by making carboxylic acids more reactive, smoothing the way for them to link up with amines. In life sciences, researchers use methods built around this chemistry to create custom peptides for exploring diseases or crafting diagnostics.
Beyond the research setting, you find this chemistry supporting the pharmaceutical world. Sometimes, speed makes a difference; scientists need reactions that work quickly and don’t leave behind lots of impurities. With 1-Carboxymethyl-Pyridinium Chloride, yields improve, and unwanted byproducts often get cut down. The value shows up down the line, with more reliable test results and medications that meet higher purity standards.
Experience Working with the Compound
Anyone who’s wrestled with sluggish reactions knows the frustration. Once, in the middle of a project on new peptidomimetics, using traditional coupling agents left us with stubborn mixtures tough to purify. Switching to 1-Carboxymethyl-Pyridinium Chloride shifted the situation—a cleaner product, higher yield, and much less frustration on my team’s faces. Keeping research moving forward sometimes depends on chemistry that just works. This compound delivers that edge.
Challenges and Considerations
No chemical comes without a tradeoff. 1-Carboxymethyl-Pyridinium Chloride works best in carefully managed environments. Water and air exposure can break down the magic it provides, so attention to detail in handling matters. Lab safety officers also highlight the need for solid ventilation and responsible disposal, recognizing potential health risks if mishandled.
Waste reduction remains a topic for every lab group. By favoring reagents like this one that produce fewer side products, researchers chip away at the growing pile of hazardous waste from synthesis labs. Professional chemists and students alike benefit from talking openly about ways to balance performance with green chemistry principles.
Improved education can make a difference. Less-experienced team members pick up safer routines and smarter waste management when projects work in a transparent, collaborative way. Many universities now blend responsible reagent selection into their courses, giving future generations a head start.
Looking Ahead
Innovation in the chemical industry moves fast, but foundational reagents like 1-Carboxymethyl-Pyridinium Chloride continue to earn their place. Whether aiming to shorten drug development cycles, chase the next big scientific insight, or train upcoming chemists, the compound acts as a practical solution again and again. As industries push for greener solutions and tighter regulations, demand for smart, reliable reagents will only rise.
Respect for Chemical Hazards Starts Before You Open the Bottle
Looking at a white powder or a clear bag, it’s easy to forget how dangerous a lab can be. Sometimes chemical names get tossed around without much thought, but 1-Carboxymethyl-Pyridinium Chloride deserves a healthy dose of respect. Its potential risks demand attention, no matter how seasoned the chemist. Rushing into it is a quick way to end up with unnecessary burns, lung irritation, or far worse.
Know Your Chemical, Know Yourself
No matter the project, the first thing to reach for is the Safety Data Sheet (SDS). Everyone in the lab should know how to access it. One glance tells you toxicity, storage rules, and what to do during an emergency. Everyone nods at the idea of “know your hazards,” but sometimes real learning happens when a bit of dust lands in the wrong place and you scramble for an eyewash. Remembering that story keeps the SDS in focus, every time.
Start with the Gear You Wear
Long sleeves, chemical-resistant gloves, and eye protection—these aren’t optional for handling something like 1-Carboxymethyl-Pyridinium Chloride. Nitrile gloves hold up better in most labs. Pull on a lab coat with closed cuffs. Forgetting goggles even once changes how you see safety forever. Inhaling dust from this compound can cause lasting damage, so wear a mask or, better yet, use a certified fume hood.
Good Workspaces Prevent Trouble
Spills get noticed in clean spaces. Chemical-resistant benches help contain problems, and a fume hood traps hazardous particles before you breathe them. Testing the fume hood’s airflow before work keeps you out of trouble. No food, drinks, or clutter in your workspace—less temptation to get sloppy. Portable fans or open windows can move dust in the wrong direction and spread the problem, so keep airflow controlled.
Don’t Wing It — Measure and Store with Care
Spoons from the kitchen don’t cut it. Use dedicated, clean spatulas and scales for transfer and weighing, then clean up before moving to the next task. Tightly seal containers right after use to keep moisture and air out. Moisture can change chemical behavior fast, sometimes making storage dangerous. Stash the compound somewhere cool, dry, and away from anything that might react—never next to acids, bases, or oxidizers.
Fight Complacency with Training and Buddy Systems
Everyone forgets details now and then, so regular training, even for basic chemical hygiene, helps. Pairing up on risky procedures makes it harder to skip steps. Set up emergency procedures and let everyone practice using showers, eyewash stations, and spill kits. Drills seem redundant until the first real spill.
Disposal is Not a Guessing Game
Leftovers and accidental spills must go into approved chemical waste containers. Dumping it down the drain endangers more than just your lab. Follow institutional or regional rules about hazardous waste pickups. Label every container clearly—confusion leads to chemical reactions that could put people and property in danger.
Speak Up, Fix What You See
If you spot someone cutting corners, say something. It takes real teamwork to keep each other safe. Staying informed, staying equipped, and keeping lines of communication open builds a safety culture that carries further than any one day handling 1-Carboxymethyl-Pyridinium Chloride.
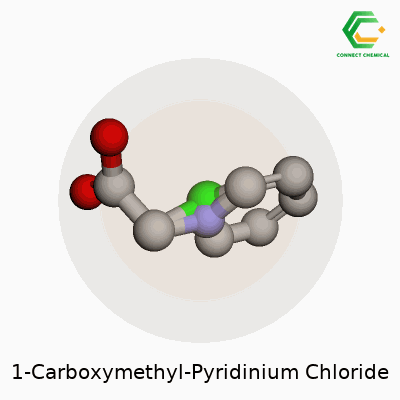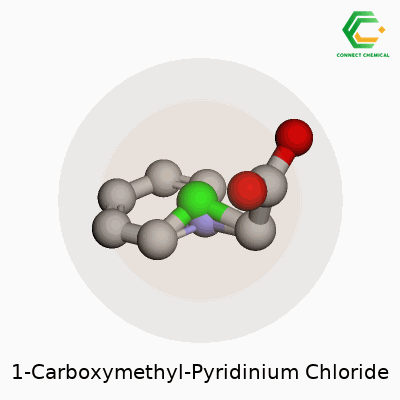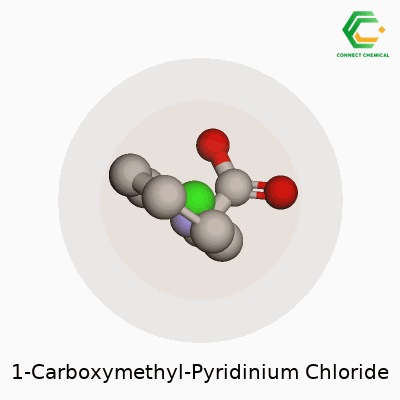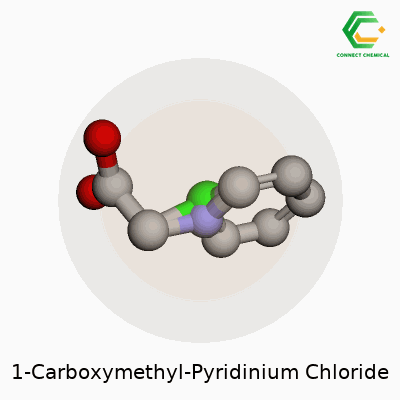
Chemical Structure: A Simple Walkthrough
1-Carboxymethyl-pyridinium chloride doesn’t get much attention outside a chemistry lab, but its physical makeup tells a useful story. Look at the backbone—pyridine, a six-membered ring where five carbons hold hands with a nitrogen. Add a carboxymethyl group (–CH2COOH) to the nitrogen spot, and now you have a positively charged nitrogen atom linked with the carboxymethyl chain. Pair this cation with a chloride anion, and that’s the full salt.
For those comfortable with chemical structures, its formula lines up as C7H8NO2Cl. Imagine the pyridinium core hosting a carboxymethyl tail on its nitrogen, charge balanced by a Cl– somewhere nearby.
Molecular Weight: How Much Does It Weigh?
Let’s break it down piece by piece. The atoms contribute to the following tally:
- Carbon (C): 7 x 12.01 = 84.07
- Hydrogen (H): 8 x 1.01 = 8.08
- Nitrogen (N): 1 x 14.01 = 14.01
- Oxygen (O): 2 x 16.00 = 32.00
- Chlorine (Cl): 1 x 35.45 = 35.45
Add them all up: 84.07 + 8.08 + 14.01 + 32.00 + 35.45 lands at about 173.61 g/mol. Chemistry students and lab techs can lean on the details, knowing this number helps with everything from basic measurements to designing synthesis routes or analytical methods.
Why This Compound Matters
Many folks move past unfamiliar chemicals, but structures like 1-carboxymethyl-pyridinium chloride play a quiet role in real work. Its polar, charged nature stirs up ideas in catalysis, electrochemistry, and separation science. With a pyridinium ring, scientists who navigate molecular recognition or phase transfer have an extra tool for controlling ionic environments.
Its solubility in water sets the stage for fast reactions. Pulling from my own days assisting a university research project, pyridinium salts often helped shuttle reactants through tougher media or acted as improvised ionic liquids. That hands-on exposure brings a layer of respect for these “simple” chemicals. They serve as scaffolds, building blocks, or ready-in-hand reagents when standard approaches get stuck.
Safety and Environmental Perspective
Anything with an aromatic ring, a nitrogen, and chloride deserves mindful handling. Chloride salts don’t seem flashy, but under certain conditions, these compounds push pH or interact with metals. In the wrong hands or spilled carelessly, environmental release brings predictable headaches. Biodegradability sometimes trails, especially with substituted aromatics.
Labs that use pyridinium compounds can make safer choices through process controls, containment, and proper waste management. Greener solvents and alternative reaction setups pop up in the literature, inviting new thinking. Chemists who value their craft dig for options before defaulting to old routines, always asking how a synthesis route can carry less baggage.
A Path Forward for Research and Industry
Creative application of compounds like 1-carboxymethyl-pyridinium chloride won’t break into headlines, but incremental improvement matters. Small groups in academic and private labs still pull these structures off the shelf, finding smarter, cleaner, and more effective ways to use them.
Knowledge grows fastest when shared widely and evaluated with care. Respect for molecular detail, transparent safety habits, and open conversations across the chemical community shape progress in meaningful ways.
Everyday Lessons from Chemical Storage
I’ve worked in different settings—industrial labs, university stockrooms, neighborhood pharmacies—and if there’s one thing that’s crystal clear, it’s that chemicals never forgive neglect. Even products that seem harmless can change character fast if tossed in the wrong spot. 1-Carboxymethyl-Pyridinium Chloride might not come up often in dinner conversation, but in any stockroom it deserves respect. People tend to think only about the dangerous powders—the ones that spark, stink, or smoke. Still, safety starts with habits, and basic storage discipline matters for every reagent.
The Real-World Science Behind Dry, Cool Storage
So many times I've seen chemical bottles sweating near steam pipes or squeezed beside windows. Heat and moisture work as a tag team, quietly causing trouble. For salts like 1-Carboxymethyl-Pyridinium Chloride, humidity spells clumping, caking, and slow breakdown. I learned from a mentor that even if a label looks fine, moisture can work inside the bottle, making warning signs almost invisible until the compound fails in a reaction or releases a strange whiff. Shelf life matters; money and time slip away once a chemical degrades before you even measure a gram.
Protecting People—and the Product—Through Smart Labeling and Segregation
I’ve watched routine safety walk-throughs catch tiny oversights—unlabeled lids, open bags, even bottles with fading ink. One overlooked sticker or missing hazard symbol, and someone new might reach for the wrong jar. With 1-Carboxymethyl-Pyridinium Chloride, it’s not just about chemical compatibility; confusion creeps in fast with any system that tries cutting corners on labeling. Deliveries come with extra paperwork for a reason. Lab managers don’t enjoy audits, but every time I’ve had to answer questions, I realized each missing record would eventually cause bigger problems.
Security Isn’t Just About Locks
I remember a time in a shared storeroom where someone left the cabinet open for ‘a second.’ Two hours later, the janitorial crew filed a report about mysterious dust and everything spiraled—surprise inspections, pointless anxiety, new safety meetings. The lesson rings loud: stay alert about access. Even if 1-Carboxymethyl-Pyridinium Chloride isn’t restricted on most lists, the rules do expect nobody grabs it as a shortcut for another product—or walks off with it. Lockable cabinets, access logs, and sign-out folders build good habits and close off loopholes.
Why Forgetting Disposal Protocol Creates Headaches
Old, forgotten bottles lead to confusion and, sometimes, real risk. Chemical inventory drift starts subtle, skips a year, and soon a dusty shelf looks like a mystery zone. Disposal takes time, yet I’ve seen headaches multiply after just a couple forgotten containers. Follow-through on disposal keeps compliance sharp and limits the chance that degraded chemicals sit around while people guess what’s inside. Environmental rules make it clear: no draining, no trash bin shortcuts. Professionals double-check disposal steps, and it’s how we set new staff on the right habits from day one.
Making It Stick Day to Day
The best systems only succeed if people own them. Relying on routine walks, checklists, and reminders sounds dull, but those habits kept me out of trouble more than once. Dry, room-temperature storage, away from direct light, works for 1-Carboxymethyl-Pyridinium Chloride. Regular checks for leaks, clear labels, no overcrowding—even the smallest chemical stash benefits. Safety and reliability grow from practical vigilance, and nobody regrets a little over-preparation once a busy week spins out of control. If you work closely with chemicals, these choices should become second nature, saving a lot of trouble down the line.
Using Chemistry Know-How to Judge Solubility
1-Carboxymethyl-pyridinium chloride isn’t a chemical you bump into in everyday life, but its solubility question matters for chemists trying to use it in a lab or manufacturing setting. Seeing “chloride” in the name helps—chloride salts often dissolve pretty well in water. The extra positive charge on the pyridinium ring and the polar carboxymethyl group only improve those odds. Chemists usually count on these types of salts to break apart and pull water molecules in, thanks to charge attraction.
In college labs, we always trusted that ionic compounds—especially those with smaller, single-charged ions—readily blended right into water. This always made solutions easy to handle and reactions more predictable. A salt like this draws plenty of attention, especially for teams hunting for water-based applications, because it checks those solubility boxes without much drama.
Testing Solvents Other than Water
People sometimes try to use organics like ethanol, methanol, or acetone as solvents when water isn’t a fit. From firsthand experience, switching from water to alcohol-based solvents gets tricky for ionic salts. Alcohols may dissolve some ionic compounds, but usually not as well as water does. And with acetone, you can almost always expect poor results. The solid usually wouldn’t budge much when mixing with acetone; you need pretty polar, hydrogen-bonding solvents for these salts.
Solvents like dimethyl sulfoxide (DMSO) and dimethylformamide (DMF) do better than acetone but don’t always reach the kind of dissolution that water achieves. Labs that try to push these kinds of compounds into non-aqueous solvents often have to warm things up or stir vigorously to get much out of it. Experience shows it’s never as straightforward as using water.
Why Solubility Should Matter
Solubility changes how people use a compound. If a chemical won’t dissolve, it creates clumpy, uneven mixtures and can slow or totally block key reactions. In industry, poor solubility kills efficiency and jacks up costs, either through waste or extra processing. Everyday products depend on solutions that stay clear and don’t separate or crystallize out over time. For researchers, unpredictable solubility means more time spent troubleshooting and less progress toward results. Mistakes can pile up, lab work gets delayed, and budgets stretch thin.
Finding Out for Sure
Even with all the signs pointing to high water solubility—ionic nature, polar side chain, and plenty of chloride salts doing the same—no chemist really trusts only textbook logic. Lab teams run simple water-solubility tests: add the salt to water, stir, check for cloudiness, and maybe measure concentration. Data from published papers and official product sheets often reveal the numbers, but not every newer or specialty chemical gets that treatment.
Companies and labs might turn to software predictions for a quick guess, but models only go so far—real-world testing caps the uncertainty. Analytical chemists with years in the field expect surprises now and then, especially when molecular interactions get complicated. It pays to review product data from trusted suppliers, check regulatory safety information, and draw on published studies for the most up-to-date insights.
Moving Toward Practical Solutions
For teams needing to work with 1-carboxymethyl-pyridinium chloride in water-based systems, things should proceed smoothly. If water poses problems, looking toward more specialized solvents like DMSO or DMF might offer a workaround, but it’s smart to test small batches first. Simple steps—stirring thoroughly, heating gently, using clean water—make a difference in the lab or in plant-scale setups.
Relying on trusted data, running hands-on solubility tests, and making sure storage conditions don’t pull water back out of your mixture all matter. My own time behind the bench always taught that watching how a chemical behaves in the real world beats any prediction. That’s the best way to keep projects on track and surprises out of the process.