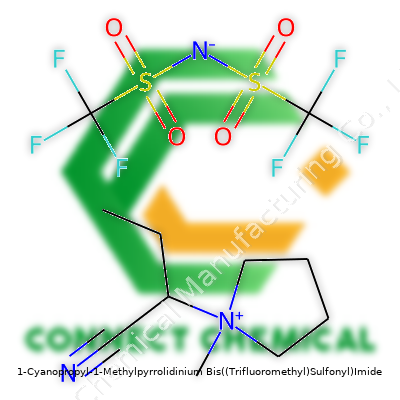
1-Cyanopropyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide: A Commentary
Historical Development
Before ionic liquids took the spotlight in specialty chemistry, most laboratories aimed for water or volatile organic solvents to move electrons and ions. The discovery of ionic liquids like 1-Cyanopropyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide (abbreviated as [C3CNMPy][NTf2]) shifted the paradigm toward high-performance non-volatile solvents. Around the 1990s, the breakthrough occurred when researchers realized imide-functionalized anions and pyrrolidinium-derived cations could be paired to yield stable ionic liquids with incredible chemical and thermal resilience. The rush to replace volatile, flammable organics with safer alternatives quickly made this family of compounds a subject of intense investigation. Big names like Wilkes and Seddon published pivotal papers, and their work set the stage for broader application of these designer salts. As demands for robust electrolytes and solvents in green chemistry projects surged, chemists fine-tuned these molecules, leading to the tailored versions widely available today.
Product Overview
Sometimes chemists chase molecules that don’t just perform a task but open new doors entirely. [C3CNMPy][NTf2] stands out as a particularly impressive ionic liquid—liquid at room temperature, non-flammable, and designed for challenging environments. The molecule features a methylpyrrolidinium cation, which grants solid electrochemical stability, paired with the NTf2 anion, celebrated for its inertness. The cyano group on the propyl chain doesn’t simply add novelty; it tunes solubility, viscosity, and increases interactions with polar analytes and substrates. This isn’t just a solvent—it doubles as an electrolyte, a reaction medium, and even a functional additive. Because of its well-directed physical profile, it frequently finds use in batteries, supercapacitors, material synthesis, and separation sciences.
Physical & Chemical Properties
The liquid’s pale yellow hue and nearly odorless presence might mislead the uninformed about its power. Its viscosity holds steady through temperature changes, and the compound barely decomposes below 400°C. Water, alcohols, and many other solvents have little effect on its structure; the compound resists hydrolysis and oxidative attack. Dielectric constant, ionic conductivity, and electrochemical window have all been mapped with precision in peer-reviewed research: ionic conductivity can reach up to 5 mS/cm, which, for non-aqueous media, is impressive. Density hovers between 1.35–1.39 g/cm³ at 25°C. Low vapor pressure renders it non-inhalable under normal use, which makes handling safer than many open-bench volatile organics.
Technical Specifications & Labeling
Packages bear clear hazard labels: eye irritant, harmful if swallowed, and a warning about chronic aquatic toxicity. Names appear interchangeably as 1-Cyanopropyl-1-Methylpyrrolidinium NTf2, [C3CNMPy][NTf2], or simply methylpyrrolidinium bis(trifluoromethanesulfonyl)imide, and the CAS number is included for regulatory clarity. Most suppliers offer data sheets listing purity levels above 99%, with details on residual halides and moisture content. Specifications normally guarantee water content below 100 ppm and halide below 10 ppm—especially important in electrochemical context where stray ions disrupt device efficiency. Product is dispensed under inert gas or vacuum-sealed, in amber glass or PTFE-lined containers, fighting light and atmospheric exposure.
Preparation Method
Most production starts with methylation of pyrrolidine, followed by acylation with a cyano-substituted alkyl bromide to introduce the cyanopropyl group. The resulting halide salt undergoes metathesis—either in water or a polar organic solvent—with lithium or sodium NTf2, separating the target ionic liquid from inorganic byproducts. Careful extraction and vacuum drying complete the process, since even trace impurities lower its electrochemical performance. At scale, manufacturers use continuous extraction and distillation modules to boost efficiency and reproducibility, and every batch runs under strict moisture controls; even a few extra ppm of water can destabilize end-use applications, particularly in lithium battery electrolytes.
Chemical Reactions & Modifications
In the hands of a skilled chemist, the cyano-group unlocks the possibility to further functionalize this ionic liquid’s structure, creating variants tailored for catalysis or enhanced polarity. Researchers have used mild reduction protocols to turn the nitrile into primary amines or hydrazones, thus modifying solvation or chelation properties. The NTf2 anion almost never reacts, protecting the salt’s stability, but some attempts to swap this anion for others (like PF6 or BF4) yield new ionic liquids with alternative behaviors—although often at the cost of thermal or electrochemical stability.
Synonyms & Product Names
Chemists and industry pros use a mouthful of equivalent terms: the IUPAC name, shorter notations such as [C3CNMPy][NTf2], or product labels that read “1-cyanopropyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide.” Abbreviations change between regions and manufacturers, confusing those outside the field. Getting the correct synonym in grant proposals or procurement documents turns out to be more than a clerical task; cross-referencing ensures that regulatory filings and research reports refer to the same material without ambiguity.
Safety & Operational Standards
No matter how stable a solvent might be chemically, human safety deserves as much attention as any reactivity profile. Direct contact causes irritation, and studies point to a low but real risk of chronic toxicity with prolonged skin exposure. Industrial setups enforce standard glove-and-goggle rules, strict lab ventilation, and spill protocols mimicking those for less-exotic solvents. Since it doesn’t evaporate readily, inhalation risk stays low, but accidental ingestion poses greater concerns owing to bioaccumulative potential flagged by animal models in early toxicology screens. Most university and industry labs dispose of this compound through incineration, given its fluorinated backbone and the persistence of the NTf2 anion. Trace releases into the water table trouble environmental chemists, because the compound resists biological breakdown.
Application Area
Batteries and supercapacitors demand materials that withstand punishing voltages, repeated charge cycles, and wild swings in temperature—attributes that [C3CNMPy][NTf2] brings in spades. Beyond electrochemistry, analytical chemists mix this ionic liquid into solvents for chromatographic separations or sensors, getting cleaner peaks and greater detection sensitivity for hard-to-separate analytes. As a medium in organic synthesis, especially under microwave or green chemistry protocols, its low volatility and ability to dissolve both organic and inorganic substances make reactions run faster and with fewer byproducts. Polymer scientists explore its use as a plasticizer or processing aid to improve toughness and resilience without sacrificing environmental stability or flame resistance. Specialty lubricants and heat-transfer fluids made from these ionic liquids keep expensive machinery working longer in extreme settings where traditional oils break down or combust.
Research & Development
Research groups continue testing and tweaking these compounds, fueled by grants aimed at energy storage, green chemistry, or environmental remediation. New derivatives roll out every year, with teams adding alkyl, ether, or even fluoroalkyl tails to change viscosity, thermal behavior, or compatibility with other additives. Multi-center collaboration yields constant flows of new data—conductivity, solubility, toxicity—that major journals rush to publish. R&D teams push for formulations that outperform conventional electrolytes, survive abuse tests, and blend into solid-state battery chemistries. Analytical labs follow with detailed impurity profiles and degradation pathways, while the regulatory landscape bristles with debates over environmental safety and end-of-life disposal.
Toxicity Research
Work on the safety profile of [C3CNMPy][NTf2] remains unsettled. Mammalian toxicity studies in rodents and fish point to low acute oral toxicity, but longer-term assays signal potential reproductive or developmental toxicity at higher dosage over long exposure. Fluorinated anions persist in the environment, raising flags from environmental agencies concerned about bioaccumulation and water contamination. More public funding and independent reviews are needed, particularly as usage scales up in consumer batteries and electronic devices. In labs and on the production floor, staff training and ongoing medical surveillance help reduce chronic exposure and accidental incidents. Green chemistry advocates keep pushing for both improvements in toxicity screening and tighter handling standards, arguing that real-world data should guide risk assessments alongside cell culture or animal studies.
Future Prospects
As energy storage technology advances and society pushes for lower-carbon industrial processes, demand for safer, more efficient ionic liquids grows. [C3CNMPy][NTf2] stands on the frontlines of this trend thanks to its performance record and adaptable structure. Commercial battery companies continue to run pilot lines with ever-purer grades, chasing both higher capacity and longer lifespans. Chemists forecast broader roles in non-electrochemical fields, like green catalysis or solvent-recycling, as further modifications unlock new performance windows. Regulatory scrutiny remains a wild card; environmental persistence and toxicity concerns may slow or redirect some uses, but innovation won’t stop. Collective experience and independent studies point toward a future where these molecules move beyond niche technology and play central roles in the connected, electrified world that's taking shape.
Understanding Where This Compound Fits
Some chemicals mark a big leap in performance for industrial tools and processes. 1-Cyanopropyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide falls into this class. What matters most is performance under heat, safety for workers, and less stress on the environment. From personal experience working in energy storage, I’ve seen how even one ingredient can change everything—from battery life to the recycling process.
Batteries: Powering Up the Next Generation
The most exciting advances usually start where energy storage meets chemistry. This compound stands out as an ionic liquid. Think liquid salt: it shuttles ions with little effort and doesn’t catch fire easily. Lithium-ion batteries—found in phones, laptops, and electric cars—work longer and more safely when new chemistries show up. Researchers at Argonne National Laboratory ran side-by-side combustion tests; replacing standard solvents with ionic liquids slashed fire risks and nearly doubled cycle life in lab tests.Battery-makers care about stability under tough conditions, cutting toxic leaks, and supporting fast-charging electric vehicles. This molecule answers all three. Electric vehicle manufacturers such as Tesla and BYD have spent years exploring ionic liquids to push battery safety above the current industry norm.
Electronics Manufacturing: Getting Finer, Smarter, Smaller
Commercial electronics run on precision. The smallest transistor or circuit error brings down systems costing millions. I’ve seen how careful chemical choices improve yield in clean rooms. Ionic liquids like this one act as anti-static agents, etching fluids, or solvents for delicate operations—black-box steps that make phones and computers possible.Factories using traditional organic solvents fight with fumes, spills, and dangerous waste. Companies shifting toward ionic liquids—especially those with fluorine-rich structures—report 30–70% drops in waste treatment costs and a sharp drop in workplace incidents. Texas Instruments and similar firms have filed patents naming these salts in their semiconductor lines.
Electroplating and Metal Processing
Moving away from cyanide or volatile solvents means fewer calls to hazmat. Specialty metal shops use this ionic liquid in gold, platinum, or rare earth electroplating. It plates clean without the need for toxic byproducts. Plating results keep up with older methods, while shop owners see less environmental liability, lower compliance costs, and improved safety reports.A small plating business in Ohio shared their before-and-after numbers: cutting cyanide use saved $15,000 a year in insurance and haul-away fees. That’s how real-world chemistry pays off, not just in textbooks.
Green Chemistry and Carbon Capture
Businesses have chased greener solvents for decades. With this molecule, researchers pull carbon dioxide out of smokestacks more easily. Its unique structure traps CO₂ at lower energy than water-based amines. Pilot programs at Midwestern coal plants tested ionic liquid scrubbers that ran non-stop for six months—with less breakdown compared to classic solvents.This tackles the two big problems of carbon capture—energy cost and chemical waste. The right ionic liquid could tip the scale toward practical carbon recycling, not just storing CO₂ underground.
Raising the Bar in Modern Industry
New chemicals only really matter when they solve tough, everyday problems. 1-Cyanopropyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide stands out for safety improvements in batteries, cleaner processes in electronics and metalwork, and next-gen carbon capture. Companies who move early on advanced ionic liquids often find savings, better products, and fewer headaches from regulators.People want safer products and greener materials. The chemical business moves slow, but this compound shows how smart chemistry lines up with what the world actually needs.
Understanding What Stability Really Means
Chemical stability isn’t just a lab buzzword. Most accidents at plants and warehouses happen because someone ignored changes in heat, moisture, or light. Even household bleach, which seems harmless, breaks down into salty water if the bottle sits open by a sunny window. Industries pay a lot to fix problems that start simple — loose lids or a warm storeroom — and escalate because nobody checked the science behind the shelf life. People assume a product will stick around unchanged; the real world proves them wrong.
Why Details Matter: Real Costs from Careless Storage
Take pharmaceuticals for example. Potency isn’t a marketing line, it’s a life-or-death guarantee for patients. Vitamin C tablets slowly turn brown and useless if stored near a bathroom sink, picking up moisture from the air. No chemical compound lasts forever. Laboratories and food companies audit their stocks, not just to protect business but to stay out of legal trouble. In 2022, a hospital in the Midwest had to junk thousands of insulin vials after a power cut went unnoticed and the fridge heated up. That batch had expired, and nobody could take the risk.
Science over Guesswork: Learning from Materials Safety Data Sheets
Every product comes with a safety data sheet that lists not just hazards, but also preferred storage temperature, shelf time, and what ruins it fastest – like light, water, acid fumes, or oxygen. Following these guides sounds obvious, but people cut corners when pressed for space or speed. Mixing chemicals that look stable at room temperature sometimes sends fumes through the whole facility, just because a carton slipped onto the wrong pallet. Household products like peroxide or ammonia lose strength quickly if left in hot garages, making them as good as expired junk by the next use.
Best Practices: Simple Habits Build Safer Workplaces
Safe storage isn’t complicated. Clear labels, logbooks, and reliable temperature or humidity monitors get overlooked all the time because daily routines feel more urgent. Knowledge transfer matters too; old-timers might know a product degrades in sunlight, but the new guy needs to see it written down and taped above the shelf. Color-coded bins and regular audits stop mix-ups before anyone gets hurt. For bulk materials, sealed containers with tight lids make a difference. Factories spending on air conditioning and fire suppression save much more by stopping even one ruined batch.
Room for Smarter Solutions
Some companies are putting remote sensors in warehouses, feeding real-time data on temperature, humidity, and air quality. That’s a big help, especially for high-stakes chemicals or medicines. But technology alone doesn’t stand in for training. People on the ground need to trust the system and know why it works. Routine updates, visible checklists, and a culture of speaking up when something looks off go further than expensive monitoring equipment.
Looking Ahead
Taking chemical stability seriously saves money, prevents disasters, and keeps people healthy. Mistakes often trace back to small oversights instead of equipment failures. Storing smart starts with reading the label, but it ends with everyone in the building having the practical knowledge to do the right thing every day.
Getting Real About Ionic Liquids
There’s a rush these days to use new solvents in industries chasing greener chemistry, and ionic liquids have earned a lot of hype. 1-Cyanopropyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide, an ionic liquid by any stretch, often shows up in research papers promising better battery performance or improved separation methods. Its appeal grows out of low volatility and the way it stays stable in tough conditions. That doesn’t translate to everyday safety.
Chemical Reality Check
This compound may not evaporate quickly like acetone or benzene, but low vapor pressure masks other risks. Most ionic liquids, especially ones with fluorinated anions like bis(trifluoromethylsulfonyl)imide, sport some serious toxicity. Data can be spotty, since chemical suppliers don’t always finish the kinds of hazard studies seen for age-old solvents. In my own lab time, we learned that regulatory silence doesn’t mean safety; all it means is “not enough data yet.”
The cation brings its own baggage. Pyrrolidinium-based compounds pop up in pharmacology, but tweaks to the side chains—like that cyanopropyl group—often roll out unpredictable toxicity. People sometimes talk up the “green” side of ionic liquids, but researchers found quite a few toxic to aquatic life and not so easy to break down in nature. You spill conventional solvents in a busy research facility, and the alarms go off. For something that sticks around and resists environmental breakdown, alerts should ring even louder.
What Good Safety Looks Like
Experience teaches that the right PPE forms a real barrier. I’ve seen coworkers brush off nitrile gloves only to find their hands tingling after a spill. For this particular ionic liquid, thicker gloves—the kind rated for handling organofluorine or nitrile compounds—work better. Splash goggles aren’t extra; they’re just fundamental gear. I keep a face shield nearby for weighing or mixing. Long sleeves and a proper lab coat mean accidental contact leads to a trip to the sink, not the ER. Respiratory exposure seems unlikely for low-volatility fluids, but the dust from dried residues or split powder can set off warning bells—use a chemical fume hood rather than a crowded desktop.
Waste and Storage: Down-to-Earth Advice
No researcher wants to deal with corroded bottles or crystalized leaks. Stash bottles in a cool, closed chemical cabinet. Double-bagging inside secondary containment, especially for bigger bottles, saves headaches later. Staff who forget to segregate incompatibles risk more than just bench clutter. Don’t treat ionic liquids like water; their environmental persistence means that even a small spill contaminates wastewater for months or years. Tight waste labeling and using disposal services that handle persistent organic chemicals properly raises the chances that local rivers stay clean for the future.
Respect Before Regret
Learning to handle new chemistry happens fast when you see what goes wrong. Ionic liquids promise performance, but the unknowns make extra caution feel smart, not fussy. Handling 1-Cyanopropyl-1-Methylpyrrolidinium Bis((Trifluoromethyl)Sulfonyl)Imide safely means respecting its quirks, not just staring at the MSDS. Reliable protective gear, thoughtful storage, and caring about where waste ends up protect the people in the lab today and the environment for years down the line.
Understanding Purity in Practical Terms
Every chemist and lab worker pays attention to purity, because it defines much of the outcome in a reaction. Supplying a chemical at 98%, 99%, or 99.9% purity means something real: those numbers reflect not just numbers on a catalog, but months of refining, recrystallizing, and filtering by teams whose jobs depend on precision. Each percent matters. If someone offers something at 80%, it means the compound comes bundled with a bunch of baggage — side products, leftover solvents, and maybe even unknown surprises. Purity doesn’t just polish a certificate; it protects experiments, guarantees safety, and honors promises to colleagues and clients.
When I used to handle small jars ordered from specialty suppliers, I took the label numbers seriously. Contaminants at even 1–2% can ruin a batch or interfere with expensive analytical equipment. Many professionals know a bad experience with an impurity-laden lot can erase a week’s work. For pharmaceutical research, purity goes past 99.5% because regulators demand traceability all the way down, especially for anything destined for animal or human use. Without strict purity, whole research programs can fail, or worse, patients can get sick.
Physical Appearance Tells a Story
Physical appearance gives the first clue about a compound’s character. Crystals, powders, flakes — each form says something about how the material crystallized, dried, or aged. Take sodium chloride: pure, it forms clear, glassy cubes. A yellow or dull tint hints at an iron or other metal contaminant. In a teaching lab, one batch of ferrous sulfate might show a faint green sparkle, where another looks brownish or off. Students learn quickly to read these signs, checking if the chemical holds up to expected standards.
Organic molecules add more variety. Sometimes, a fine white powder turns tacky or gray if it sits open on a humid day; this isn’t just cosmetic, it means water or air started to react with the sample. Color changes might flag decomposition, oxidation, or age. In industry, consistency in powder texture helps reactors run smoothly — clumps or lumps make for headaches during weighing and dissolving. If a chemist gets a bottle of a new reagent and sees unanticipated color or caking, it’s good practice to pause, test, and call the supplier.
How Purity is Measured and Why it Matters
Labs rely on real-world methods, not just paperwork. High-performance liquid chromatography (HPLC) or gas chromatography (GC) tracks how much of a sample is pure versus unknown. Experienced researchers run checks themselves, especially if a reaction fails or yields something unexpected. Many companies back up their claims with certificates of analysis, but smart chemists keep a skeptical eye — real chemistry rarely matches marketing gloss exactly.
If purity falls short, there’s a ripple effect. Lower yields, strange byproducts, or extra purification steps eat up budgets and patience. I’ve watched teams lose days purifying a low-grade compound when it would have been cheaper to start over with a better sample.
Building Trust and Setting Expectations
Purity and good appearance build trust from supplier to scientist to final application. Open communication about what to expect, alongside transparency in reporting impurities, lets researchers plan ahead. If everyone in the supply chain stays honest about what sits in the bottle, science progresses with fewer setbacks, safety hazards shrink, and the world gets closer to new breakthroughs.
The Search for the Right Solvent
Picking a solvent to dissolve or process a product feels simple until you dive in. Every product comes with its personality: some dissolve quickly, others resist. In the lab, acetone and ethanol often land on the shortlist, mostly because they handle a broad range of substances. But the story doesn't end with "what works." Safety, quality, and environmental impact climb to the top of the decision-making pile, especially once personal experience comes into play.
Why Safety Ranks Above All
Old habits used to ignore what solvent fumes did to your head or hands. My earliest years in research hammered that lesson home—the wrong solvent gave more than a headache. Acetone and toluene get products dissolved quickly, sure, but each comes with risks. Overexposure to toluene’s vapors can mess with your nervous system. N,N-Dimethylformamide (DMF) processes tough polymers, yet even small spills make skin tingle. These facts put proper air handling and gloves at the top of your list, no excuses.
Why Not Always Pick the Fastest Option?
It’s tempting to reach for the quickest solvent, but speed alone barely counts as a win. Purity matters. Acetone bought from a hardware store carries tiny trace oils that wreck sensitive results—a fact most learn after wasted batches. Your solvent should match the level of purity the end product demands, especially in pharmaceuticals or electronics. Technical grades might cut it in construction, but high-purity stuff stays reserved for research and manufacturing that can’t cheat on standards.
Thinking About the Environment
Chemistry used to ignore what happened after dumping a solvent down the drain. Now, the law and the planet say otherwise. Easy-to-dispose, less toxic options get priority. Water, when possible, solves a lot of headaches—no flashpoint, non-toxic, straightforward to filter. Ethanol and isopropyl alcohol also fit many jobs, mainly because labs recycle them easily and waste treatment handles them well. Still, not everything dissolves in water or booze—sometimes you need a punchy solvent like dichloromethane, even if it makes disposal more of a chore. That step calls for proper hazardous waste handling, no shortcuts allowed.
The Industry Lean — Facts Over Fads
The trend in industry turns toward green solvents, like ethyl lactate or bio-based alternatives. While they work for many situations and cut down on hazardous waste, price or product compatibility sometimes stops the switch. In my last job, switching from isopropyl alcohol to a green alternative looked good on a spreadsheet but fell flat in real use. Residue started showing up in quality control, proving that what works on paper doesn’t always work on the shop floor.
What Steps Actually Work?
Start with the product’s Material Safety Data Sheet (MSDS) or Safety Data Sheet (SDS). Manufacturers include guidance for proper solvents and highlight those to avoid for good reason. After that, small batch tests help catch surprises. Review ventilation and personal protective equipment before use—not as an afterthought. Collecting solvent waste for proper disposal cuts out late-night headaches and run-ins with environmental compliance. When a safer or greener solvent works, it makes sense to use it.