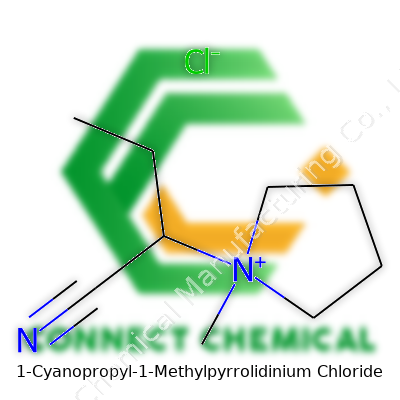
Exploring 1-Cyanopropyl-1-Methylpyrrolidinium Chloride: Insights, Applications, and Advancements
Historical Development
The world of ionic liquids came a long way from dusty chemical benches to today’s packed R&D pipelines. Chemists have chased more stable and functional ionic materials since the early days of quaternary ammonium compounds in the mid-twentieth century. Research in the past few decades found a new kind of promise in pyrrolidinium-based salts, and 1-Cyanopropyl-1-Methylpyrrolidinium Chloride landed on the map thanks to synthetic versatility and room-temperature stability. Laboratories in Europe and East Asia first experimented with the pyrrolidinium core to overcome volatility and flammability issues in earlier generations of ionic liquids. Group discussions over coffee in academic corridors slowly translated into peer-reviewed publications, which then spurred commercial interest. Collaborative work between academic and corporate researchers has steadily shaped this compound into a candidate for demanding applications like advanced electrolytes and green solvents.
Product Overview
1-Cyanopropyl-1-Methylpyrrolidinium Chloride stands out from the crowd of ionic liquids by combining robust cationic frameworks with a functionalized anion. This salt takes on a solid or viscous liquid form at room temperature, showing high solubility in polar solvents. Unlike some traditional salts, it doesn’t release pungent fumes or corrosive vapors in laboratory settings, making it accessible for both industrial-scale processes and academic research. As a result, the chloride variant makes a suitable intermediate for further chemical manipulations or as a template in novel material synthesis. Chemical suppliers distribute it in sealed, moisture-free containers to maintain integrity and ease of storage. Spec sheets typically detail batch provenance, purity levels typically exceeding 98%, and molecular weight values, supporting traceability for regulated industries.
Physical & Chemical Properties
The compound appears as a white to pale yellow powder or viscous liquid, with a molecular formula of C9H17ClN2 and a molecular weight hovering just over 188 g/mol. Its low melting point and good thermal stability boost its usefulness across various temperature regimes. Most ionic liquids face scrutiny regarding their hygroscopic nature, but this one resists ambient moisture more effectively than older ammonium analogs. Conductivity reaches respectable values at room temperature, which helps explain its emerging role as an electrolyte in next-generation batteries. The cyano group along the propyl chain grants extra polarity, making the substance miscible in a wide range of polar organic solvents. Researchers consistently measure electrochemical window values above 3 volts in non-aqueous systems, widening its use in energy storage. With low volatility and a density ranging around 1.05–1.09 g/cm³, accidental inhalation risk remains very low during handling.
Technical Specifications & Labeling
Industry standards for labeling include specific catalog and lot numbers, clear molecular structure depiction, and full hazard labeling compliant with GHS. Concentration and purity figure prominently on the label, reflecting each batch’s chemical fingerprint—every percent above 98% makes a real difference in high-end research. Some suppliers go further by adding barcode tracking and supply chain documentation, which trace each container from production to delivery. Besides molecular weight, melting and boiling points, and solubility data, labels feature recommended shelf life and strict storage guidelines to avoid hydrolysis or side reactions. In regulated spaces, the import and handling conform to international standards like REACH in Europe and TSCA in the United States, confirming every shipment meets environmental and human safety criteria.
Preparation Method
Chemists synthesize 1-Cyanopropyl-1-Methylpyrrolidinium Chloride through quaternization reactions, often starting with N-methylpyrrolidine and a 1-cyanopropyl halide derivative. The reaction usually runs in a polar aprotic solvent—acetonitrile and N,N-dimethylformamide are common—under nitrogen to avoid unwanted oxidation or hydrolysis. Anhydrous conditions prove essential; moisture doesn’t just knock down yields but can also lead to byproducts that complicate purification. After the quaternization, the product crystallizes out or forms a viscous oily phase, which allows for decanting and subsequent washes with cold ether or ethyl acetate. The chloride salt purifies further by recrystallization or column chromatography, depending on the final application. Final drying under vacuum at moderate temperatures strips residual solvent, a small but necessary step for reliable results in downstream chemistry, especially where trace contaminants can skew electrochemical performance.
Chemical Reactions & Modifications
Innovative chemists continue to explore the reactivity of the cyano and pyrrolidinium moieties. The cyano group can undergo nucleophilic additions or reductions to produce amines and amides, unlocking pathways to entirely new families of functionalized ionic compounds. The pyrrolidinium ring displays remarkable resistance to hydrolysis but participates in alkylation or sulfonation strategies that tweak solubility and ionic strength. Researchers in academic and industrial labs treat the chloride salt as a platform, swapping the counterion out for less nucleophilic or more hydrophobic alternatives. For example, ion-exchange columns quickly substitute chloride with bis(trifluoromethane)sulfonimide or hexafluorophosphate, giving the resulting compound different solvation and electrochemical behaviors. Modifying the length of the N-alkyl chains or changing their substitution pattern also transforms the physical state, persistence, and compatibility with polymers or inorganic frameworks, which proves vital for customizing the compound for energy storage or catalysis work.
Synonyms & Product Names
This compound sometimes pops up in catalogues and academic articles under names like N-methyl-N-(3-cyanopropyl)pyrrolidinium chloride, 1-methyl-1-(3-cyanopropyl)pyrrolidinium chloride, or simply as MCPPC. Some labs refer to it by shorthand product codes or proprietary names offered by chemical vendors, which can complicate literature searches but offers insights into growing specialization in chemical supply. Product identifiers often tie back to CAS numbers for regulatory documentation—these numbers follow a strict registry system to avoid confusion between international labs sourcing from different suppliers. Awareness of alternative nomenclature proves essential for early-stage researchers checking for precedent in patents, ensuring comprehensive understanding of the intellectual property landscape in this chemical class.
Safety & Operational Standards
Regulatory frameworks set clear procedures for the handling and disposal of pyrrolidinium-based salts. Direct skin contact gets avoided through use of standard PPE—nitrile gloves, lab coats, and eye protection. Fume hoods remain the venue of choice for scrupulous chemists who weigh out dry powder or dissolve the material in organic solvents; even though vapor risk sits low, microparticulate can irritate airways or eyes on careless handling. The compound rates low flammability and volatility, but rigorous documentation persists for storage and spill clean-up, reflecting persistent vigilance against accidental exposure in shared lab environments. Waste streams carrying residual salt get tracked and neutralized before leaving the premises, conforming to local and international hazardous waste rules. Training sessions and clear signage within chemical storage rooms reinforce these habits—chemists learn from experience and repetition, not just safety data sheets filed away in cluttered drawers.
Application Area
Innovation takes this compound across industries, driven first by its proficiency as an ionic liquid. Electrochemical device developers—fuel cells, batteries, capacitors—value it for broad electrochemical stability and high ionic conductivity, seeking materials that boost energy density and lifespan without sacrificing recyclability. The cyano functionality opens up catalytic possibilities, where the compound operates as both solvent and reactant in organic transformation reactions. Materials scientists and polymer chemists blend it into composite electrolytes, taking advantage of improved mechanical and thermal properties for solid-state battery work. Specialty purification tasks, like removal of specific metal ions from wastewater, tap into unique solvation properties granted by the N-alkyl-pyrrolidinium scaffold. Even pharmaceutical research dips in, exploiting the salt as a green solvent in reaction screens for drug precursor synthesis, since its low volatility and chemical inertia simplify separation and product isolation. Each researcher chases new frontiers—hoping the next experiment reveals advantages overlooked by others.
Research & Development
Ongoing research tracks every incremental improvement in this class of compounds. Multidisciplinary teams at universities and corporate labs continually optimize synthesis, streamline purification, and invent new derivatives. Interest accelerates among electrochemical engineers designing batteries for electric vehicles, with prototypes featuring pyrrolidinium-based electrolytes competing against historic stalwarts like imidazolium salts. More sustainable production practices—the search for greener solvents and lower temperature reactions—form a lively subfield. Collaborative consortia band together to map material property landscapes using big data and machine learning, correlating structure with performance. If a research group stumbles on an improved preparation method or reaction, that tactic soon becomes a workshop favorite, repeated and refined in labs across the globe. Success spreads out in conference presentations and preprint uploads, inspiring other groups to dream bigger and push technical barriers further.
Toxicity Research
As the compound enters more industrial and consumer-adjacent markets, toxicity researchers scrutinize every pathway it may follow through ecosystems and workplaces. Acute exposure studies in rodents and cultured cell lines examine cytotoxicity, organ distribution, and metabolic fate. These investigations suggest low acute toxicity at practical exposure levels, but chronic studies dig deeper to account for cumulative effects—especially if the material slips into water sources or air through accidental releases. Biodegradation and environmental persistence become hot topics for teams working with regulatory bodies, who push for full lifecycle assessment. Reports to date reveal limited bioaccumulation but prompt careful risk assessment before scaling up use in open systems. Publicly available databases, like those maintained by the European Chemicals Agency, ensure transparency and broader scrutiny beyond corporate risk managers. In practice, these guidelines drive responsible manufacturing, regular medical monitoring for exposed workers, and emergency plans built into facility protocols.
Future Prospects
The chemical industry feels the pressure to carve cleaner, safer, and smarter materials for tomorrow’s critical technologies. 1-Cyanopropyl-1-Methylpyrrolidinium Chloride represents one step in the journey, with chemists and engineers betting it will anchor next-generation battery designs, greener synthesis, and circular economy processes. Both academic and industry leaders keep a close eye on this field, hunting for breakthroughs in efficiency, cost, and long-term safety. Efforts focus on expanding the design window—longer chains, new counterions, smart blends with polymers or nanomaterials. The compound’s ability to merge function and stability promises years of vibrant research and industrial competition. If the experience of the past decades holds true, collaborative spirit and honest reporting will drive the community toward safer, smarter, and more sustainable use of pyrrolidinium chlorides, echoing the call for materials that match humanity’s technical ambitions with real-world responsibility.
A Closer Look at a Specialized Chemical
1-Cyanopropyl-1-methylpyrrolidinium chloride doesn’t exactly roll off the tongue, but it holds a spot in the growing field of ionic liquids. I first heard about this chemical during a factory walkthrough with a team of engineers eager to cut costs and environmental impact in one shot. It popped up in a conversation about cleaner, safer processing options. Over several years, as industries dig for versatility and efficiency, compounds like this slip into more conversations and quietly change the way things are done.
The meat of its use lies in its role as an ionic liquid. These salts don’t behave like the crystalized table variety. They stay liquid at near room temperature, making them handy in all sorts of chemical processing. You walk through a plant, see big drums with strange labels, and somewhere in there sits a liquid salt doing quiet work. Regular solvents evaporate, create hazards, and stink up warehouses. Ionic liquids like 1-cyanopropyl-1-methylpyrrolidinium chloride avoid a lot of those issues.
Why Choose This Compound?
Plenty of solvents get the job done, but not all keep things as clean or as energy-efficient. This chemical stands out because it doesn’t flash off into the air. Factories don’t have to worry so much about emissions into the neighborhood. Less evaporation means less lost material and fewer headaches over local regulations. In my own experience, managers like the word “safer” almost as much as “cost-effective.”
The environmental crowd has an eye on processes that use greener options, and this is where the compound comes in strong. Its low volatility means plants run with fewer spills and less cleanup. The gear used to scrub fumes gets a break, electricity bills drop, and the workplace stays healthier. Not every chemical can claim this many wins just by switching out what’s in a tank. I’ve worked on pilot projects where swapping in these newer liquids dropped workplace exposure by a chunk and helped a company prove it was serious about being a good neighbor.
Real-World Applications
This pyrrolidinium-based salt steps into areas like electrochemistry and catalysis. Companies use it as an electrolyte in batteries, turning chemical energy into electrical energy reliably and safely. Besides energy storage, it appears in separation processes, where companies pull apart chemical mixtures more efficiently. Research labs have tinkered with it to fine-tune chemical reactions, making cleaner end products from complex starting materials. I've watched colleagues experiment with this compound, sometimes swapping out old, harsh organic solvents for this one and seeing gains in both worker comfort and final product yield.
The Road Ahead and Smarter Solutions
The march toward cleaner chemicals won’t stop here. While no solvent is perfect, the value of shifting to options like 1-cyanopropyl-1-methylpyrrolidinium chloride keeps adding up, especially as regulations get tougher around emissions and waste. In my work with process engineers, pushing for these swaps meant not only winning points on environmental reports but also building a team culture proud of small victories in safety and sustainability.
Progress hinges on both chemistry and willpower. Researchers continue to push the envelope, improving how these ionic liquids are produced, recycled, and used. Teams that put in the effort up front end up with smoother operations, a healthier workspace, and sometimes, a nice savings line at the end of the quarter. The shift doesn’t happen overnight or without cost, but seeing workers breathe a little easier or hearing about a battery that lasts longer tells me this route is worth the effort.
Understanding the Formula
Every molecule has a story tucked inside its atoms. 1-Cyanopropyl-1-Methylpyrrolidinium Chloride sounds like a tongue-twister, but its formula makes things clearer: C9H17N2Cl. Breaking that code, you see it contains nine carbon atoms, seventeen hydrogens, two nitrogens, and a chloride ion holding the charge. For researchers and people in materials science, tracking down the right chemical identity isn’t about showing off some fancy label — it’s about accuracy, safety, and reliable results.
Molecular Weight Matters
Anyone who’s spent time weighing out chemicals in a lab knows one thing: even a small wrong move throws everything off. For 1-Cyanopropyl-1-Methylpyrrolidinium Chloride, the molecular weight lands at 204.70 g/mol. That number isn’t just a reference; it’s the foundation for measuring concentrations and doses. Try mixing up a reaction without a precise formula and molecular weight — nothing works as intended.
Why Chemical Precision Isn’t Just for Chemists
Some folks might think details like formulas only matter to people wearing lab coats all day. The truth turns out different. Even a basic battery, a medicine, or a cleaning agent can include molecules that start conversations between atoms. Get the formula wrong, and a promising recipe becomes a danger or a dead-end. Trust earned by a compound’s formula and weight reaches far — from researchers searching for safer solvents to regulators focused on product safety.
Reliability Shaped by Education and Experience
Plenty of my time in the lab got spent double- and triple-checking numbers like these. It’s not paranoia — it’s real-world wisdom. Chemical reference books and databases, like PubChem or the NIST Chemistry WebBook, have strict review standards to help scientists dodge mistakes. Classroom education might cover formulas, but the real test comes with a scoop of powder on a balance, getting the exact grams for solutions. These details shape every result downstream, whether you’re trying to synthesize a drug or develop a new type of electrolyte for a battery.
Facing the Challenge of Mislabeling and Confusion
Errors in chemical labeling stick around as a quiet hazard. A bottle with a close-but-incorrect name, or an outdated CAS number, easily causes a dangerous mix-up. Companies and research labs improve safety and teamwork by keeping up-to-date records and labeling, plus locked-down digital systems for inventory. Tools like QR codes and barcode scanners have made tracking easier, shrinking the space for human error. For those who take shortcuts, a single mix-up proves costly, both financially and in terms of trust.
Better Solutions for the Future
Education and digital technology light the path forward. Investing in refresher training for everyone who works with chemicals doesn’t just protect people — it saves money and improves results. Databases need regular audits for accuracy. Companies benefit from encouraging a culture where people pause to double-check a label, formula, or weight. By building off solid chemical knowledge, that simple molecular formula can become the starting point for safe innovation, not a source of worry.
Understanding What You’re Dealing With
Every lab adventure starts with learning the ropes. Handling chemicals like 1-cyanopropyl-1-methylpyrrolidinium chloride is no different. This salt isn’t something most folks have on their shopping lists, but material like this shows up in specialty synthesis and research work. If you’re using it, you are likely deep in the weeds of ionic liquids or shooting for something niche in organic chemistry. Knowing how to treat this chemical well makes you more confident at the bench and keeps everyone safer.
Hazards in Real-World Terms
I’ve spent years in research labs, and experience says never trust a name alone. Don’t be lulled just because this isn’t some notorious acid or solvent. The cyanopropyl tail means someone with a slipshod approach can wind up in trouble. Studies flag eye, skin, and respiratory irritation as clear risks. Dust can fly. If this stuff lands on your skin, it can burn—gloves and a lab coat go from being “extra” to absolutely necessary. Splash goggles aren’t just for those terrible organic chemistry videos, either—they matter, because eye exposure brings risk you don’t want.
Someone once tried to hand me a vial straight out of their glove-free grip—don’t do that. I always recommend going beyond minimal PPE. Nitrile gloves, proper coat, and splash glasses are basics, not suggestions. Good science pushes us to look closer, so safety data sheets turn into a trusted resource. They don’t just live in a three-ring binder. I always checked them before pipetting a drop.
Best Storage Practice Isn’t Optional
Leaving specialty chemicals out on the bench overnight is a rookie mistake. I’ve learned the hard way: even if the bottle says "moisture stable," that’s not a license to ignore cap-tightening or storage calls. 1-cyanopropyl-1-methylpyrrolidinium chloride draws water from the air, clumping and breaking down if you let it. Moisture in the bottle means experimental results go sideways. That chemical goes straight back to a cool, dry space—away from acids and bases, away from direct sunlight, and with the lid snapped on.
Temperature swings don’t just create condensation; they can shift the chemical profile. In shared lab fridges, mark containers clearly, use secondary containment, and separate from reactive substances. This keeps unwanted reactions at bay and helps during audits—nobody wants compliance headaches over sloppy organization.
Respect Goes a Long Way
I started out assuming an unfamiliar reagent would be “like salt.” That’s just wishful thinking. Chemicals like this demand respect at every stage. Training new staff means running through hands-on protocols. Dry hands, goggles on, and always working in a fume hood. I remind newcomers that clean workspace and labeling isn’t just tidy—it prevents poisonous mistakes. These habits build trust among staff and keep small lab incidents from turning into emergencies.
Reliable risk management—the sort required by every serious lab—leans on clear procedures. No one is above those rules, from the greenest intern to the most senior chemist. Regular review of inventories, routine air monitoring if powder handling is frequent, and chemical hygiene plans all pay dividends. Problems find you when you lower your guard. My labs never had to call emergency services, and that wasn’t luck.
Room for Improvement
Better packaging, accessible real-world training, and up-to-date safety data keep labs safe. Manufacturers and suppliers should work with end-users. Provide concise hazard info and offer hands-on demonstrations when possible. Labs should invest in updated chemical management software to track everything down to the lot number. Cultivate an environment where safety isn’t a chore—it’s an everyday reality.
Looking for the SDS for 1-Cyanopropyl-1-Methylpyrrolidinium Chloride?
Anyone dealing with chemicals, especially in research or manufacturing, always faces the classic hunt for reliable safety data. In my own experience bouncing between academic labs and small tech firms, hunting down proper documentation can drag a project for days. Suddenly, even routine work with a compound like 1-Cyanopropyl-1-Methylpyrrolidinium Chloride turns into a treasure hunt. The safety data sheet (SDS) isn't just paperwork. It tells you if you need thicker gloves, extra ventilation, a special fire extinguisher. It lays out what to do after a spill or an accident, even how to store the stuff so you don’t find yourself with a mess down the road.
You’ll often find that established suppliers like Sigma-Aldrich, TCI, Merck, or ChemSpider provide downloadable SDS files as soon as you enter the product name or CAS number. ResearchGate sometimes delivers scanned copies, especially if working with niche chemicals. Public databases like the European Chemicals Agency (ECHA) or the U.S. National Library of Medicine’s PubChem also offer accessible SDS entries, though availability can lag for newer or rarely used substances.
Sometimes, though, searches turn up nothing. I remember combing through supplier pages late at night, only to find vague references backed up by zero documentation. It’s not a minor inconvenience. Without a genuine SDS, accurate risk assessment slips through your fingers. Lab safety drifts into guesswork, and insurance policies may hang in limbo. Colleagues in university labs have run into this problem so frequently, they now call the vendors. They demand SDS on delivery before opening bottles. Many times, they get the documents emailed directly from customer service teams.
Why Bother? Here’s What an SDS Actually Means for You
It’s tempting to treat an SDS as bureaucratic noise—until something goes sideways. With 1-Cyanopropyl-1-Methylpyrrolidinium Chloride, as with countless other chemicals from the ionic liquids family, hazards often fly under the radar. There isn’t always decades of peer-reviewed toxicity data to lean on. I’ve watched people trust their memory or past experiences with similar chemicals, only to learn the hard way. SDS contents are built on regulatory standards like OSHA or REACH, which pull from experimental data, legal requirements, and reported incidents.
Labs should keep digital and hard copies of every SDS on-site, ideally compiled in a way that any worker (new or seasoned) can find in less than a minute. Digital inventory tools like Quartzy or ChemInventory help, but old-school printed binders by the door still win during an emergency. Manufacturers and distributors also need to hold up their end: posting current, detailed SDS as soon as products hit the market. The reality is, a single missing document can compromise every layer of a lab safety protocol.
Building a Culture That Values Safety Data
Chasing down the SDS for 1-Cyanopropyl-1-Methylpyrrolidinium Chloride might seem annoying, but it’s not just about ticking a box. Fostering a workplace where people expect to see paperwork and pause work if it’s missing always beats running with blind trust. I’ve seen both cultures. Only one keeps people safe over the long haul. Don’t settle for silence from suppliers or mysterious bottles with faded labels. Build relationships with vendors who respond quickly, share documents on demand, and explain what’s in the sheet if you ask. Every step toward better access strengthens the whole safety net for everyone working with chemicals.
Why Purity Grades Drive Real Decisions in Labs and Industry
Anyone in chemical synthesis, research, or engineering circles eventually weighs the question of purity. In my time working with ionic liquids and specialty reagents, purity levels often become the dealbreaker—especially for ionic compounds like 1-cyanopropyl-1-methylpyrrolidinium chloride. The smallest trace of an unintended substance can swing reaction results, skew analytical measurements, and sometimes sabotage an entire project.
Standard Purity Levels Found in Reputable Sources
The typical purity for 1-cyanopropyl-1-methylpyrrolidinium chloride hovers between 95% and 99%. Most suppliers focus on the upper end—at or above 98%—for research and high-end applications. That extra few percent may sound like nitpicking, but in the chemical world, each decimal point carves away trouble. At 95%, residual solvents, unreacted starting material, or minor byproducts can interfere with sensitive reactions like catalysis. Jump to 99% and those ghosts in the mix all but vanish.
I’ve learned through long hours in the lab how much frustration stems from overlooked trace impurities. That’s the moment someone in the group re-orders a compound with a higher purity grade. Suddenly, the side reactions settle down, product yields stabilize, and analytical NMR spectra clean up. It’s a reminder that sometimes, pure means peace of mind.
Grades Serve Different Crowds: From Bulk to Analytical
Chemical suppliers usually label 1-cyanopropyl-1-methylpyrrolidinium chloride with a few grades—technical, laboratory, and analytical. Technical grade often lands at the lower end of purity and sees most use outside of critical science, mostly in larger-scale, less-sensitive procedures. The cost drops, but expectations drop too: some haze or discoloration in the product, maybe batch-to-batch irregularities.
Laboratory grade sits in the middle. Most educational or basic research projects select it because it strikes a balance: high purity, but not excessive for everyday synthesis. Analytical grade raises the bar—usually above 99%. I always pick this grade if the experiment can’t afford noise from impurities, especially with precision analytics, trace determinations, or instrument calibration.
How Purity Gets Measured
Responsible researchers need verifiable data—so I never just trust a label. Vendors provide an accompanying certificate of analysis listing chromatography and spectroscopy results. High-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), and sometimes infrared (IR) spectroscopy identify both the principal compound and lingering side substances. For an ionic liquid like this, water content represents another challenge; even tiny amounts lower shelf life and performance.
Quality Control: Improving Access and Safety
Companies and universities don’t just obsess over purity out of habit. Regulatory bodies such as REACH and the EPA impose rules to protect workers and the wider environment. A high-purity product means less chance of unanticipated toxicity or harmful byproducts down the line. For people overseeing a storeroom or dispatching chemicals across borders, tighter quality control cuts down on rejected shipments, fines, or wasted resources.
Labs can invest in their own purification—yet experience tells me there’s no substitute for buying the cleanest possible stock. Automation, better analytical tools, and third-party verification help close the gaps. Still, human eyes on a certificate of analysis remain an indispensable checkpoint.
Making Smart Choices: The Reality of Budget and Application
Research budgets put pressure on every purchase. It’s tempting to select a cheaper, lower-purity chemical and hope for the best. Yet spending a little more upfront for higher purity often pays off with time saved, fewer failed reactions, and unambiguous data. Talking through application needs with a trusted supplier saves grief—someone who actually understands these pitfalls, instead of pushing whatever stock is at hand.