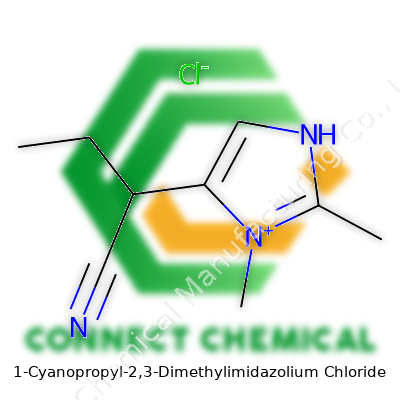
1-Cyanopropyl-2,3-Dimethylimidazolium Chloride: In-Depth Commentary
Historical Development
Chemistry tends to hit milestones when researchers set out to answer practical challenges. Over recent decades, as industries searched for cleaner solvents and specialized synthesis tools, imidazolium-based compounds—especially ionic liquids—attracted attention. 1-Cyanopropyl-2,3-dimethylimidazolium chloride did not appear out of nowhere; it came along after many years of work on the imidazole ring and its diverse substitutions. Early studies explored alkylation of imidazoles in the mid-20th century, with the push from both academic labs and industrial players eager for novel materials and catalysts. Through meticulous design, scientists moved from simple alkyl substitutions to functionalized ones like cyanopropyl, aiming to add new solubility features and reactivity.
Product Overview
Talking about this compound means looking at a room-temperature ionic liquid, where 1-cyanopropyl and dimethyl modifications create unique features. The chloride anion gives a crisp halide element for applications that need ionic character. Quaternized imidazolium salts like this one offer more than just a straightforward ionic liquid; developers can toss them into organic synthesis, electrochemistry, and even emerging battery technology. Users get a material shaped for chemical research and manufacturing, with consistent batch-to-batch performance. What stands out is the functional group on the side chain: the nitrile grants this salt strong dipolar interactions, boosting its solvent abilities and helping to solubilize stubborn organic and inorganic species that standard reactants leave behind.
Physical & Chemical Properties
Touching this compound reveals a solid at room temperature—crystalline, usually white or off-white, and highly hygroscopic. The presence of the nitrile group shifts the melting point upward compared to its non-substituted cousins. It's often readily soluble in polar solvents, especially water. From a chemical point of view, the imidazolium core resists many types of degradation, while the chloride anion lets it serve as a phase-transfer catalyst or as a medium for electrochemical deposition. It's thermally stable enough to handle moderate heat applications, yet its chemical resistance does not give way to strong bases or nucleophiles under ordinary conditions. The dipole moment jumps with the nitrile, allowing researchers to tune solvation environments for demanding applications in catalysis or separation science.
Technical Specifications & Labeling
Manufacturers tend to specify purity above 98%, confirmed through NMR, HPLC, and elemental analysis. Labels show chemical structure, warnings about moisture sensitivity, and storage suggestions: dry, airtight, out of direct sunlight. Transport and hazard information appear for regulatory compliance, given that many buyers work under GLP or ISO-certified systems. Density, melting point, pH in solution, and solubility in key solvents round out the documentation. Responsible suppliers include safety data that outline proper procedures for handling, personal protective equipment, and spill management.
Preparation Method
Lab synthesis kicks off from 2,3-dimethylimidazole, which reacts with a 1-cyanopropyl halide under controlled temperature and pressure, often in the presence of a base to capture evolved acid. Getting the chloride version usually relies on direct quaternization or through a halide-exchange protocol, finishing with meticulous washing and recrystallization steps. It takes skill to limit byproducts and get the right crystal habit for reliable downstream use. Each batch needs testing for residual starting materials and moisture, as that impacts both storage and performance in sensitive reactions. Standard operational routines don't skip quality control; researchers work closely with chemists to tighten specs for high-purity applications.
Chemical Reactions & Modifications
Chemists working with 1-cyanopropyl-2,3-dimethylimidazolium chloride quickly see the range of transformations possible. The nitrile end participates in nucleophilic additions, reductions, or even cyclization reactions, which lets users build bigger molecules off the ionic liquid. The core cation can survive mild to moderate acids and bases, and it participates in Fukuyama and other organocatalytic processes. For surface chemistry, grafting the cation onto polymers grants new materials for ion exchange, membrane technology, or advanced solid-state electrolytes. Chloride allows for anion exchange reactions vital in fields such as sensor fabrication or controlled release formulations. Not only does this compound serve as a backdrop, but, in capable hands, it steps up as an active player in the synthetic toolkit.
Synonyms & Product Names
Over time, different catalogs and publications have referenced this molecule in several ways. The full IUPAC designation spells out each functional group, though labs might shorten things to "Cyanopropyl DMI-Cl" or "N-cyanopropyl, 2,3-dimethylimidazolium chloride." Those ordering from chemical suppliers spot synonyms like "1-(3-cyanopropyl)-2,3-dimethylimidazolium chloride" or brand-designated product numbers. Accuracy in labeling matters—mix-ups cost time and money and make regulatory checks a headache if ambiguity creeps in. Clear communication along the supply chain avoids confusion, especially as regulatory oversight on specialty chemicals only tightens.
Safety & Operational Standards
Safety teams approach this compound with respect, even though the chloride salt nature might trick students into thinking it's less harmful. Gloves, goggles, and fume hood use stand as non-negotiables. Highly hygroscopic behavior means labs keep it sealed tight: accidental water absorption can change its mass and reactivity in a snap. Skin or eye contact brings irritation risks. Inhalation pathways stay closed thanks to solid form, but fine powders still demand dust minimization. Waste solutions require neutralization and collection according to hazardous material handling rules. Training helps prevent slips, and safety officers rigorously review any new application or upscaling plans. Storage sits away from incompatible chemicals and out of heat or humidity, extending shelf life and keeping reactivity constant for each use.
Application Area
A slew of industries rely on the flexibility and utility of this ionic liquid. Organic syntheses often lead the charge, as chemists appreciate tunable solvent systems that also work as catalysts or stabilizers. Electrochemical cells tap into the ionic conductivity and electrochemical window this salt offers, especially for next-generation lithium batteries or capacitors. Environmental sectors explore it for CO2 capture, seeing gains in solubility and selectivity. Advanced material science incorporates these salts into membrane matrices for new separation technologies. Researchers also put this compound to work designing innovative pharmaceuticals or drug delivery constructs, counting on the tailored solubility and reactivity profile for better formulation results. Startups even hunt for green alternatives to traditional solvents, eyeing ionic liquids as a less volatile, recyclable option. Each use case brings its own set of technical demands and regulatory restrictions, pushing technical teams to learn and adapt quickly.
Research & Development
Academic circles buzz with papers exploring new roles for substituted imidazolium chlorides. Every tweak to the side chain opens new possibilities in catalysis or molecular recognition. Partnerships between universities and the private sector push methods for greener synthesis routes, yielding less waste while conserving energy. Some projects test these salts in pilot-scale processes, learning where the compound holds up and where it falls short. R&D teams use advanced computational models to predict new structural variants that might outperform current options. This compound becomes a test bed for fundamental chemistry—molecular modeling, solvation studies, and electron transfer mechanisms all find fertile ground here. Where a real breakthrough shows up, it often stems from gritty, repeated trials and honest reporting of both successes and failure points.
Toxicity Research
Understanding toxicity shapes deployment. Early assessments screen for acute contact hazards, and—like with most imidazolium-based ionic liquids—the main issues surface at concentrated exposure, ingestion, or environmental contamination pathways. Animal studies highlight that some cationic species disrupt membrane integrity at high concentrations, raising alarm bells for unchecked industrial discharge. In aquatic toxicity tests, chloride-based ionic liquids show moderate persistence unless broken down under specialized treatment. Since trace residues can impact cell cultures or bioprocesses, labs rigorously test for cytotoxic effects before approving any pharmaceutical or biotech use. Emerging research checks for chronic exposure effects, backing up or questioning scale-up decisions thoughtfully. Regulatory agencies press for clear disclosure and ongoing commitment to best practices as more data comes in.
Future Prospects
What happens next connects strongly to both capability and responsibility. Clean energy storage demands non-volatile, non-flammable electrolytes, opening doors that this compound can walk through. Carbon capture and storage, as regulations bite down year-on-year, shine a spotlight on functionalized ionic liquids. If cost drops, these salts could move out of just specialty research markets and reach broader manufacturing sectors. Green chemistry pushes developers to design energy-efficient, low-toxicity preparation routes, closing the loop on waste and byproducts. Automation and AI-driven material discovery pick up the pace, helping scientists isolate next-generation candidates faster. By staying honest about both benefits and risks, and keeping communication lines with regulators open, researchers and manufacturers decide just how central 1-cyanopropyl-2,3-dimethylimidazolium chloride will become in tomorrow’s chemical landscape.
Getting to Know the Compound
1-Cyanopropyl-2,3-dimethylimidazolium chloride sounds like something out of a lab filled with bubbling beakers and long equations on the blackboard. At its core, this compound comes from the wider family of imidazolium-based ionic liquids. Chemists and chemical engineers lean heavily on ionic liquids because of their unusual mix of stability, low volatility, and strong ability to dissolve different types of molecules. With those features, these liquids have carved out a practical place in industry.
Pushing Sustainable Chemistry
The standout trait for 1-cyanopropyl-2,3-dimethylimidazolium chloride comes down to its primary use: acting as a solvent and reaction medium in advanced organic synthesis and catalysis. Today’s push for greener chemistry draws from the fact that traditional organic solvents often create environmental headaches. Most of us wouldn’t want to drink water out of a river downstream from an old plant that dumped volatile organic compounds because, more often than not, those chemicals stay in the environment.
Scientists began turning to ionic liquids, including this imidazolium salt. They stick around at room temperature as liquids. They hardly evaporate and rarely ignite, so air and fire safety improve. In research and industry, the focus lands on their ability to help chemical reactions move along faster or deliver more final product, all with less mess to clean up. The cyanopropyl group on this imidazolium cation tweaks its properties just enough to handle stubborn or sensitive reactions. The chloride anion helps dissolve a wider range of reactants, especially in complex or fragile chemical systems.
How Chemists Use It
Nobody makes this compound for kitchen cleaning supplies or perfume. Its home shows up in specialized labs, doing challenging jobs — like enabling carbon-carbon bond formation, extracting rare earth elements needed for smartphones, or enhancing biomass conversion to biofuels without relying on toxic solvents. Hydrogenation, alkylation, and other central steps in the fine chemicals and pharmaceuticals sectors benefit from its presence, where traditional routes stall out or cost too much. With the right ionic liquid, a sluggish reaction can become much more practical.
I spent time in a university lab working on catalytic processes for greener plastics. We kept returning to ionic liquids when classic solvents like toluene gave us headaches from both yield and safety perspectives. One grad student almost ruined her shoes with a careless splash of dichloromethane. After swapping to an imidazolium salt, our yields went up, and the spills vanished since the liquid clung to the beaker instead of vaporizing or flowing across the bench.
Room to Grow, Challenges to Face
No chemical brings a free lunch. The price and complexity of synthesizing 1-cyanopropyl-2,3-dimethylimidazolium chloride mean that bulk chemical companies don’t snap it up by the ton. Waste handling also demands strict oversight, even if emissions into the air drop off. Future solutions hinge on simpler recycling pathways for ionic liquids and finding renewable feedstocks for their production. These steps play into a broader shift toward making industrial chemistry not only effective but responsible.
As the world expects cleaner tech and safer chemicals, compounds like 1-cyanopropyl-2,3-dimethylimidazolium chloride provide stepping stones. People in industry and research keep shaping how such specialized tools get from the lab bench into factories and, eventually, into safer products for everyone.
Real-World Experience Shows Why Storage Matters
Anyone who’s spent time in a lab knows the feeling: hunting through shelves for a bottle that’s lost its label, or opening a container to get hit with a strange odor that means the contents turned unstable. Chemical storage isn’t about ticking off a checklist—it’s about keeping people safe, the science reliable, and resources protected from waste. Most incidents I’ve seen happened not because of some wild experiment, but because of small mistakes in handling and storage.
Stable Compounds Still Demand Smart Storage
Consider a common lab staple like acetone. Acetone evaporates easily, and just leaving the cap loose can do more harm than you think. Not only does it disappear into the air, polluting indoor spaces, but it also gets contaminated quickly. Something as simple as a tightly sealed bottle—stored away from heat and direct sunlight—keeps it pure and safe. Storage in a cool, well-ventilated spot goes a long way for most organics and solvents.
Humidity, Light, and Temperature Create Problems
Many compounds hate moisture. Hydrolysis can turn a helpful reagent useless, sometimes dangerous. I’ve seen anhydrous salts cake up and become impossible to use after just a day outside their desiccator. Light can ruin certain chemicals, especially light-sensitive dyes or pharmaceuticals. These go in amber bottles or behind UV-blocking doors—simple materials from the supply room often make all the difference.
Labeling: The Small Step That Prevents Big Trouble
Clear labeling seems obvious. Yet in practice, old hand-written labels fade, or worse—go missing. More than once, confusion led to someone grabbing the wrong substance. Keeping dates and batch numbers legible reduces mistakes. A permanent marker and a strip of masking tape can prevent project delays and safety scares.
Segregation Prevents Reactions Gone Wrong
Stories circulate about acids and bases sharing a shelf, only for one bottle to spring a leak. Mixing strong oxidizers with organics can lead to fires. I once saw a rusted cabinet door—a reminder that fumes from improperly stored acids corrode everything nearby. Chemical storage rules call for segregation, but it’s also about using some common sense: treat incompatible substances like feuding neighbors. Give them space and strong walls between.
Learning From Oversights, Not Only Manuals
Regulatory documents (like OSHA, NFPA codes, or Safety Data Sheets) offer a starting point. Yet, habits learned from co-workers or firsthand observation have shaped my habits just as much. For example, stacking bottles two high saves space, but sooner or later, someone drops one. Storing heavy containers on lower shelves takes no extra effort, and avoids broken glass.
Simple Solutions Bring Big Improvements
Investing in vented storage cabinets improves air quality and keeps flammables secure. Desiccators and refrigeration units work well for materials that struggle with moisture or heat. Safety showers, spill kits, and eye washes belong close to where chemicals live. No complicated technology is needed—attentive housekeeping and a culture of safety do more than fancy automation.
Building a Safer, Better-Run Space
Safe and careful storage leads to better science and fewer worries. Checked labels, proper containers, and separate shelves save budgets and keep emergencies rare. Colleagues go home healthy, work doesn’t disappear because of a fume or a spill, and learning or production keeps moving forward. There’s always one more habit to pick up, but respect for storage does more than just tick boxes—it keeps the lab or workspace running.
What Purity Really Means for Everyday Choices
Products rarely come in a single, one-size-fits-all version. Lab chemicals, supplements, even everyday kitchen ingredients get offered in a spectrum of purity levels. At first glance, this might sound like corporate upselling, but purity shapes real-world outcomes. Tiny traces of impurities make a huge difference, whether you're running industrial machinery, mixing medicine, or even just home brewing a special batch of beer.
During a college internship, I worked in a research lab. We bought regular supplies for basic cleaning—think isopropyl alcohol or acetone—off the shelf. For experiments, even a speck of dust counted as contamination. Researchers demanded exact percentages. A lower grade meant more unknowns, more wasted batches, and more headaches. At the time, the fuss seemed overblown. Now I see how skipping careful choices turned neat plans into messy reruns.
Purity Grades: More than Marketing
Not all jobs need a product that’s been purified within an inch of its life. Take sodium chloride. Table salt sits in everyone’s kitchen. Food-grade salt goes through rigorous checks for safety—enough for seasoning dinner—but not the level chemists want for drug synthesis. Pharma manufacturers search for “USP grade” or “reagent grade” salt, paying extra to ensure invisible contaminants don’t fake out their results or risk patient safety.
The food industry uses different levels for the same ingredients. Breweries chase away off-flavors by picking higher-purity malt extract or flavorings. Bakers rely on flour free of additives for sourdough. In each case, asking about purity isn’t about elitism—it saves time, keeps processes predictable, and lets people sleep easier at night knowing their work holds up to scrutiny.
Impact of Overlooking Purity
Mixing up purity ratings creates real costs. Hospitals once delivered tainted heparin because suppliers cut corners. Even a tiny slip in purity let contaminants into the market, putting lives at risk. Stories like that show why regulators demand Certificates of Analysis for drugs and chemicals. Consumers don’t always see those checks, but the consequences of skipping them add up quickly.
In home projects, ignoring grade differences doesn’t always bring tragedy—sometimes it just means a botched loaf of bread or patchy paint. But the bigger the stakes, the more each percentage point in purity matters. I remember one woodworking class where an unlabeled solvent ruined a finish because it wasn’t pure enough. Annoying for a hobbyist, but devastating if it happened on a production line or in a medical device factory.
Clearer Labels, Smarter Choices
Transparency in labeling helps everyone. Consumers deserve to know what grade of product they buy, without needing a chemistry degree. Companies could move away from subtle branding and push out clear, honest explanations: “lab grade,” “food grade,” or “industrial grade”, right next to every item name. Retailers can post comparison charts so buyers spot the difference at a glance.
Buying what you need—the right purity, not just the cheapest or most available option—leads to fewer failed batches, fewer health risks, and a whole lot less waste. That’s one area where experience, regulation, and plain old common sense line up. Everyone wins when purity matches purpose.
Why Chemical Safety Can’t Be Ignored
Everyone who has worked in a lab, a plant, or even used strong cleaners at home knows accidents happen fast. All it takes is one spill, one splash, or a moment of forgetting goggles, and things go from calm to chaos. So handling hazardous chemicals isn’t only for scientists in white coats. Living with these risks means treating safety as a daily part of the job.
Getting To Know What You’re Using
Every bottle, powder, or solution comes with its own dangers. Hydrochloric acid burns skin, ammonia fumes wreck lungs, acetone catches fire in a blink. Labels and Safety Data Sheets (SDS) break down what to expect. None of that fine print is for show. Updates in these documents reflect accidents people already learned the hard way. So, before pouring, scooping, or mixing, the best idea is to read what’s in front of you and actually believe what you’re reading.
Personal Protective Equipment Actually Matters
Throwing on a pair of gloves or a lab coat isn’t just a checklist item for inspectors. I’ve seen coworkers flick a glove off and discover too late that the chemical finds even the smallest cut. Nitrile gloves stand up to acids better than latex. Some fumes go right through surgical masks—only respirators keep out dangerous vapors. Rubber boots, face shields, and splash goggles turn close calls into forgettable stories instead of emergency room visits.
I remember one summer job at a water treatment plant. A guy ignored instructions and wore shorts—he wound up in urgent care with chemical burns. No one wants to dress for the job they don’t want, but skin and lungs don’t heal the way bruised pride can.
Storage and Labeling—No Shortcuts
Stacking chemicals on any empty shelf invites trouble. The wrong acid next to the wrong base, or a solvent near a heat source, can blow up more than your day. Flammable liquids need cool storage, strong acids and oxidizers don’t belong in wooden cabinets. Mixing up similar looking bottles because labels rubbed off led to one of the closest calls I’ve seen—a mix that started foaming and fuming before it even reached the sink.
Permanent markers and fresh labels don’t cost much. Leaving chemicals in old soda bottles, unlabeled spray canisters, or anything not meant for storage has ended up as a headline more often than seems possible.
Never Go It Alone With Unknowns
The temptation to clean up a small spill or sniff a container to guess what’s inside runs deep for anyone wanting to impress the boss or save time. It feels awkward to admit you don’t know how to do something safely. Yet, nearly every serious chemical accident starts with that one small decision. Getting a coworker’s attention, hitting the eyewash for a false alarm, or stopping work to Google the right procedure beats the alternative.
Culture Over Compliance
Most solutions don’t involve complicated systems. People need to speak up, ask questions, and not get mocked for being careful. Access to the right equipment and regular reminders keep safety active, not just words in a training PowerPoint. Investing in a culture where nobody feels silly for double-checking means everyone goes home whole at the end of the day.
Respect for chemicals doesn’t mean fear, it means understanding not just your own limits, but the limits of the people around you. That’s how serious injuries get prevented—one sensible choice at a time.
The Everyday Reason for Technical Questions
Anyone who has worked in research, clinical settings, or manufacturing encounters questions like, "Can you provide the molecular weight and chemical formula for this product?" Maybe it’s a chemist preparing reagents for a project or a pharmacist reviewing documents for a regulatory submission. These questions might sound technical, maybe even routine, but their impact stretches further than the lab bench. Every result, safety sheet, and claim comes back to what’s actually in the bottle.
Traceability and Trust in Practice
Knowing the molecular weight and chemical formula isn’t about satisfying curiosity. These numbers are a basic form of quality control. For example, in my years managing lab inventories, mistakes cropped up when two nearly-identical chemicals sat next to each other on a shelf. Only a clear chemical formula could spot the difference before a wrong substance entered a crucial solution. A few missing grams in molecular weight might look harmless for a test tube mix, yet could spell disaster if scaled up for a production batch or dosed by a healthcare provider.
Proper labeling, complete with molecular weight and formula, isn’t just bureaucracy. It’s part of a system that lets facilities track every step from source material to finished product. Imagine trying to trace the source of a contamination outbreak with incomplete records — without these details, identifying the actual culprit turns into guesswork. Regulators, like the FDA or EMA, frequently demand this information for just that reason. They know that broad declarations don’t suffice if patient safety is in the equation. An error of a few decimals in molecular weight can change dosage calculations entirely, risking overdose or underdose, especially in pediatric or highly sensitive therapies.
The Supply Chain and Testing Challenges
Supply chains for chemicals stretch across continents and shift rapidly whenever shortages or shipping delays hit. Without a unifying standard referencing the chemical formula and exact molecular weight, buyers have a hard time comparing products between suppliers. I’ve watched purchasing teams waste days trying to decode whether the same product from different catalogs matches up, all because one company listed only “active ingredient” and the other included full molecular data. This slows down research, increases costs, and opens the door to costly mix-ups.
Clarity Builds Stronger Science
Full chemical identification supports reproducibility. One team’s results in Boston can only be matched in Singapore if both are using the same species of molecule, not just something with a close-enough name. Less ambiguity means fewer failed replications and surprises when products land in the hands of end users. Journals, grants, and regulatory submissions now routinely require precise chemical information and batch documentation. None of this comes from a craving for detail alone. It’s about ensuring that results hang together beyond the boundaries of one laboratory, campus, or country.
Fixing the Gaps
Making life easier boils down to habits. I’ve found that suppliers who share detailed specifications right on the product labels or online listings earn more customer trust in the long run. Smart labs keep chemical inventories digital and enforce checks so no shipment enters without all identifying info matched — formula, weight, batch. A no-compromise stance on documentation helps projects move faster and makes problem-solving easier when things go off track.
Final Thoughts
Sharing molecular weight and chemical formulas isn’t about paperwork for paperwork’s sake. It’s how the industry builds trust, maintains safety, and makes science reliable. Pushing for open, standardized chemical information doesn’t just help regulatory teams; it supports the whole cycle, from discovery to application, and keeps everyone a little safer along the way.