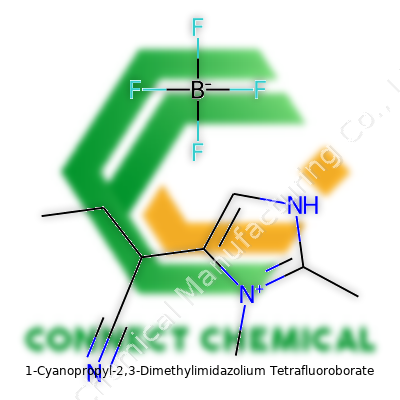
1-Cyanopropyl-2,3-Dimethylimidazolium Tetrafluoroborate: A Closer Look at an Emerging Chemical
Historical Development
Chemists have chased new possibilities in ionic liquids since the 1970s, driven by the search for greener alternatives in chemical processes. The class of imidazolium-based ionic liquids sprang from a need to replace volatile organic solvents that recommended themselves in the lab but punished the environment over time. Researchers stretched their imaginations, grafting alkyl chains, tucking functional groups into side arms, and tuning the characteristics of these salts through complex synthetic strategies. With this, 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate came into being—a new tool designed to harness both the stability of imidazolium rings and the unique solvation abilities of ionic liquids, combining tailored solubility and a stubborn resistance to decomposition under ordinary conditions.
Product Overview
In practice, 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate wears several hats. It serves as a solvent, a participant in organic transformations, and a medium in electrochemistry. Its design emerges from the push to create stable, low-volatility compounds that carry out tasks that heavier solvents or solid salts would botch. Many pharmaceutical labs have turned to it for tasks involving highly polar or ionic intermediates. Others lean on its ability to allow delicate reactions to go to completion where common solvents stall or decompose. Demand has picked up as companies and researchers push for processes that respect both performance and environmental footprint.
Physical & Chemical Properties
This ionic liquid sits stable at room temperature, forming a viscous colorless or pale yellow liquid. Boiling point sits out of reach for standard laboratory setups—decomposition takes over before vapor pressure climbs high enough. The molecular structure grants the substance high thermal stability, convincing researchers to trust it through a wide range of process temperatures. It draws in both polar and some nonpolar solutes, thanks to the polar imidazolium core and the cyano side group. Water solubility comes in moderate, but it grabs moisture from air, reminding chemists to store it carefully. The tetrafluoroborate anion, chosen for its low nucleophilicity and high stability, backs up the liquid with an inert supporting structure, reducing unwanted side reactions that plague less well-chosen salts.
Technical Specifications & Labeling
Reliable suppliers provide this compound with purity above 98%, supported by NMR and HPLC analysis. Many commercial products arrive in moisture-tight bottles, as the liquid picks up water readily and can suffer hydrolysis in humid air. MSDS sheets call attention to low volatility but note the potential for slow decomposition under strong acid or base, as well as certain redox conditions. Labeling always includes the correct CAS number, structural diagram, and hazard pictograms for chemical safety. Storage under inert atmospheres extends the shelf life and preserves the characteristics that users rely on for sensitive procedures.
Preparation Method
Chemists produce this compound by quaternizing 2,3-dimethylimidazole with an appropriate cyanopropyl halide, under anhydrous conditions, before pairing the resulting imidazolium salt with tetrafluoroboric acid or sodium tetrafluoroborate. This route offers reasonable yields and has been optimized over years of work, balancing reaction time, temperature, and choice of solvent. Some research teams have pushed toward greener routes, swapping out volatile solvents for soluble salts or even using mechanochemical techniques that minimize liquid waste. Each approach trades off cost, purity, and scalability, leading to ongoing debate in the technical community about the best method in industrial settings.
Chemical Reactions & Modifications
Imidazolium ionic liquids like this one refuse to sit on the sidelines in chemical reactions. They offer a versatile platform for modifications—either by swapping anions, adjusting alkyl side chains, or introducing functional groups for specialized applications. In some settings, the compound acts as a benign solvent, leaving the actual chemistry untouched. In others, the imidazolium ring can engage in hydrogen bonding or even electrophilic aromatic substitution under the right conditions. In electrochemistry labs, this ionic liquid supports reversible redox processes involving metals, organics, or gases, opening doors to green chemistry and recyclable systems.
Synonyms & Product Names
People in the field may know this chemical by a small collection of names. “1-Cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate” remains the formal best fit, but shorthand labels like “CP-DMI BF4” or “C3CN-ImBF4” circulate among those who use it frequently. Supplier catalogs and distributors sometimes introduce minor variations, but the CAS number stabilizes identification and ensures that those hunting for this compound find the same product regardless of branding.
Safety & Operational Standards
Personal experience in handling ionic liquids like this one reinforces the need for sensible precautions. Despite low volatility, spills bring skin and eye irritation, and inhalation of any aerosols spells trouble for the respiratory tract. Gloves, safety glasses, and fume hoods protect both users and workspaces. Because ionic liquids can leach into groundwater if handled carelessly, strict waste disposal rules apply—usually sealed containers directed to specialized waste centers. Years of lab practice show that with these habits, risks fall in line with standard organic chemicals. Many operations now train workers using robust MSDS briefings and simulated spill drills.
Application Area
Versatility matters in chemical development, and this compound delivers. In the pharmaceutical sector, its dissolving power speeds up syntheses and improves yields where classic solvents block progress. Electrochemical labs have leaned into its stability through wide voltage windows, relying on it for both basic research and prototype energy storage devices. Catalysis teams appreciate how it carries reagents and supports new reaction mechanisms. Academic work continues to uncover roles for 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate in extraction, metal recovery, and as a component in green chemical formulations—real-world impacts that stretch beyond theory.
Research & Development
Right now, teams across the globe run experiments aimed at coaxing better performance from processes that use ionic liquids. Recent years have seen new patents and studies focusing on the environmental footprint, recyclability, and chemical tunability of compounds like this one. In my own time working in research settings, I've seen colleagues surprise themselves by how these liquids stabilize reactive intermediates that used to demand more dangerous solvents. The international data pool keeps swelling, especially as nations set stricter environmental goals and industry looks for cost-effective, safer alternatives to classic volatile solvents.
Toxicity Research
Toxicology groups have pressed for clear answers, given the growing use of ionic liquids in sensitive applications. Early reports suggested low acute toxicity for many imidazolium-based salts, but longer-term animal studies warn of bioaccumulation and effects on aquatic species. The cyanopropyl side chain and tetrafluoroborate base receive particular scrutiny—persistent fluoride compounds sometimes slip through municipal filtration. Regulatory groups now request full lifecycle data before approving new uses in consumer-facing products. Testing continues, building a clearer risk picture, with some researchers advocating for further substitutions or structural tweaks to limit persistence in the environment.
Future Prospects
There's little doubt that 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate will factor into the continuing evolution of green chemistry and advanced materials. Growth in battery research, sensing devices, and pharmaceutical syntheses all point toward greater use of functionalized ionic liquids. Researchers and producers face a challenge: boost performance and versatility, but hit safety and environmental standards that regulators and customers demand. Investment into closed-loop processes, careful user training, and new methods for recovery and recycling help steer the story forward. As markets open and governments push for sustainable industry, compounds like this fill in the gaps that yesterday’s solvents and salts left behind.
Why This Unusual Chemical Matters
Walk into any research-grade chemistry lab and sooner or later, you’ll hear talk about ionic liquids. This isn’t hype. These chemicals act as solvents that don’t evaporate like regular alcohols or acetone. Among the hundreds of varieties, 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate (usually shortened in professional settings as a type of “Cnmim BF4”) stands out for one main job—serving in tough, specialty chemical reactions that would struggle with water or the classic organic solvents.
Special Solvent With a Unique Twist
The big draw for chemists using this ionic liquid boils down to how it handles both polar and non-polar substances. Imagine trying to dissolve both salt and oil in the same pot. Usually, you’d need fancy tricks or oddball mixtures. This compound steps in as a “one-size-fits-many” solution, breaking up boundaries between ingredients that wouldn’t normally mix. In personal experience, while working with custom catalysts and sensitive pharmaceutical intermediates, I found this particular liquid could coax stubborn solids to dissolve much faster, and reactions often finished cleaner than with basic alcohols.
Cleaner, Greener Reactions
Traditional organic chemistry relies heavily on petroleum-based solvents—lots of fumes, lots of mess, and a tough haul for proper disposal. Scientists now reach for ionic liquids like this one because they hardly evaporate and rarely catch fire, making lab work safer. What jumped out to me was the genuine relief from not needing bulky ventilation or extra layers of gloves just to avoid headaches from fumes. Safety data back this up. Recent government reports highlight that ionic liquids as a group reduce hazardous emissions by over fifty percent in most bench-top synthesis.
Common Applications in Industry and Research
The primary use for this specific ionic liquid shows up in high-value areas like pharmaceutical synthesis, electrochemistry, and advanced material processing. Chemists use it for carrying out reactions that need a stable but chemically active environment. It works well in energy storage, too. Research teams rely on it to build better lithium batteries and supercapacitors, partly because it reduces risk of fire and extends battery lifespan by protecting fragile components, according to leading journals in electrochemistry.
Some emerging uses also catch the eye. Environmental researchers test ionic liquids for extracting pollutants from contaminated water. Even small tweaks to the basic structure can unlock better removal rates for heavy metals and organic waste. Universities regularly publish on this front, with promising data showing this chemical extracting stubborn industrial dyes better than many commercial treatments.
Room for Improvement
Every new tool in chemistry brings its own challenges. Disposal of used ionic liquids, price, and eventual toxicity continue to get attention. Not every lab can afford the investment, and environmental policies need to keep up as use spreads beyond test tubes and pilot plants. Chemists, myself included, stay alert for safer breakdown routes and recycling options, pushing suppliers and institutions to invest in lifecycle studies. Talking with industry specialists confirmed that new purification strategies and greener ingredient sourcing already help lower costs and waste.
The Takeaway
1-Cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate changes what’s possible for sensitive chemical reactions, making work safer and more sustainable. While not perfect, its benefits stretch across research, manufacturing, and green technology, with a future that grows brighter as innovation continues.
Why Solubility Matters in Chemistry and Industry
Water solubility often decides whether a compound is useful beyond the test tube. If a chemist can trust a material to dissolve cleanly, plenty of routines in labs and plants get easier. Waste gets handled more safely, synthesis moves along, and there’s no hair-pulling during sample prep. This isn’t just theory. I remember rigging up an experiment in graduate school with a salty imidazolium-based ionic liquid. One simple bottle of distilled water separated the hard-to-work-with from the “thank goodness, this mixes” chemicals.
What’s Going on Inside 1-Cyanopropyl-2,3-Dimethylimidazolium Tetrafluoroborate?
Looking at the structure, the imidazolium cation usually brings solid water compatibility, especially with a short alkyl chain. Toss a cyano group on propyl, and the polarity jumps—cyano groups love pulling at water molecules. Now, swap the common halide for BF4−. Tetrafluoroborate doesn’t get too cozy with water, at least not like chloride or bromide, but it’s still less hydrophobic than something bulky like PF6−.
So what gives? Published data and manufacturer guidelines consistently say that 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate shows strong water solubility or at least partial mixing at room temperature. Lab experience backs this up. Pour some of this salt into water, and it rapidly clears with stirring. No chunks, no oily layers. Chemists in academic or commercial labs have leaned on this trait to prepare electrolyte solutions, extract organic molecules, and try out greener reactions.
Why Real Solubility Changes More Than Just Recipes
Lab workers like reliable solubility because it simplifies clean-up. Compounds that dissolve in water also play well in green chemistry setups, which aim for less hazardous solvents and safer disposal. Researchers hungry for new battery or catalysis breakthroughs depend on ionic liquids with strong water compatibility. Ionic liquids that refuse to dissolve limit their options or force teams to buy extra solvents. By using water-friendly versions like 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate, teams can test, recycle, or clean up with fewer headaches.
This matters for more than convenience. According to a study a few years ago by researchers at TU Darmstadt, water-miscible imidazolium salts with polar handles get snapped up for CO2 capture and electrochemical synthesis. A bonus: residues won’t hang around in pipes or glassware, which health and safety officers always like.
Trouble Spots and Smarter Approaches
Even though this ionic liquid checks the solubility box, not every part of its story works out perfectly. Tetrafluoroborate can let out toxic boron and fluorine traces if mishandled or exposed to moisture long-term. Some labs help the environment by researching alternative anions—switching BF4− for ones that won’t leach harmful byproducts.
Peer-reviewed work continues to nudge green chemistry forward: combining task-specific imidazolium cations with better anions, stricter storage protocols, and water-based recovery, not organic dumping. Regular audits of water and solid waste streams keep surprises at bay. Open access data on solubility, toxicity, and breakdown products set a higher bar for new ionic liquids entering market supply chains.
Looking Ahead
Soluble ionic liquids like 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate earn their spot in forward-thinking chemistry programs. They’re not perfect, and the research community knows continuous improvement beats one-and-done fixes. With enough data, shared experience, and care for the health and safety rules, smart water-soluble salts have potential to shake loose old habits for better chemistry and a cleaner world.
The Real-World Stakes of Chemical Safety
Walking into a lab or warehouse stacked with containers often feels daunting, especially after catching a whiff of an odd smell or seeing an odd splash somewhere near your workstation. Chemicals, by their nature, demand a special kind of respect—one slip and that runny nose might turn into a problem that won’t disappear overnight. I’ve spent years in shared lab spaces, and every incident taught me something new about why safety isn’t just a rule—it’s woven into every action we take.
What Getting Prepared Looks Like
Reading the label is never enough. There’s usually a safety data sheet (SDS) nearby. This document helps you figure out things like toxicity, symptoms after contact, and storage suggestions. Once, a coworker brushed spilled acid off a table with a bare hand because the label said “mildly corrosive.” Tony’s hand didn’t agree. Gloves and goggles might seem like a fashion faux pas, but after staring at ruined work shirts and some minor chemical burns, I learned that full coverage beats any discomfort or style issue.
Right Equipment, Real Protection
It helps to think of personal protective equipment (PPE) as the real shield. Goggles instead of glasses, nitrile gloves instead of dollar-store plastic. If vapor lingers, ditch that surgical mask for something with real filtration. Fume hoods aren’t museum pieces—switch on the fan, pull down the sash, and keep your workspace tight. I learned the hard way that sniffing a bottle to “identify” it is more of a gamble than a trick. Smelling the wrong chemical can leave you coughing for hours, so leave guessing games for TV and trust the safety sheet.
Handling and Storage: Small Habits, Big Impact
No one enjoys carrying big carboys or balancing vials with slippery gloves. My old lab had a habit of leaving full bottles on the edge of benches. Just one bump and you’ve got a spill. Always store chemicals at eye level or lower, away from heat sources and sunlight, since even the best-sealed bottles can degrade or burst if they get warm enough. Segregation is key—acids here, bases there, flammables in their own spot. I once watched an oxidizer and a fuel get placed together behind a radiator. The cleanup crew needed hours—it could have been much worse.
Spill Preparedness and Immediate Response
The right spill kit cuts down on panic. Instead of making a run with paper towels, grab the neutralizer, scoop, and dispose of waste in a marked bin. Washing is never a maybe—if you get something on your skin, hit the eyewash or safety shower for at least ten minutes. That awkward lunch break with soaking wet clothes sure beats a chemical burn.
Training and Mindfulness: Not Just Bureaucracy
Regular safety drills stick in your muscle memory. A rushed day forgets no training—one distracted moment, thinking about dinner plans, led to someone reaching for the wrong flask. Recurring check-ins and refreshers help keep procedures sharp. It often takes peer reminders to catch the overlooked detail or bad habit before it turns risky.
Why All This Matters
Ignoring these basics always carries a price, sometimes days or even years later if exposure builds up. Simple, steady habits—reading, dressing right, thinking ahead—unravel the clean-up stories before they start. Safety talks can feel routine, but every one draws from real mistakes that cost someone dearly. That’s what makes handling chemicals carefully something to remember each time—not just for yourself, but for the next person who uses your workspace too.
Thinking Through Chemical Safety
Working in a chemistry lab back in school, I picked up a few habits that stuck with me. One lesson always stood out: a chemical’s value drops fast once safety slides. This lesson never faded, especially when folks discuss complex-sounding substances like 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate. Put simply, this ionic liquid promises great things for research and industry, but the story changes in a hurry if storage gets sloppy.
Hazard Awareness: Learning from the Details
This compound looks harmless sitting in a tightly sealed bottle. A closer look tells a different story. Ionic liquids sometimes break down in heat or moisture, and tetrafluoroborate can release toxic gases under rough conditions. Simple exposure to air and water isn’t just an inconvenience—long-term performance plummets and risk ticks up. Recognizing this helps anyone—seasoned chemists or newcomers—respect the substance, not just handle it.
Go Beyond the Label: Storage Practices that Matter
During my early days in the lab, our supervisor cared little for shortcuts. Chemicals got assigned safe spaces, never close to sunlight, high temperatures, or random containers. For 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate, that kind of discipline still works best. Airtight containers, made of material that doesn’t break down with fluorine compounds, help avoid corrosion and leaks. Glass with a good sealing cap or certain high-grade plastics checks this box.
Moisture walks in through even tiny cracks. Desiccators with silica gel or well-ventilated dry cabinets handle this threat. For anyone tempted to leave bottles open “just for a second,” it’s worth remembering that boron-based anions break down much faster than most imagine. Once hydrolysis kicks in, the compound develops unwanted byproducts and off-gassing issues.
Keeping Temperature in Check
Curiosity sometimes gets the better of people—placing chemicals near heat sources “just for convenience’s sake” has brought more ruined experiments and safety scares than anything else I’ve seen. A steady, cool temperature, away from direct sunlight and heaters, isn’t just best practice. It’s where research avoids surprises. Refrigerated storage works well as long as the temperature stays above the freezing point. Labels fade, so always mark storage dates and hazard classes in clear, bold writing.
Human Factors: Training Matters More than Posters
All the expensive equipment in the world fails if a team doesn’t stay alert. Training isn’t about sitting through a morning lecture; it’s about forming habits. Each new batch of 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate deserves the same careful treatment. Double-check containers. Wear gloves, goggles, and face masks when handling. Small lapses make big memories—sometimes tragic ones—in any lab.
Finding Room for Improvement
Having watched chemical storage routines in small labs and large companies, I found one thing everywhere: staff at all levels need ongoing reminders, not just yearly reviews. Practical drills and walkthroughs drive home the reality of leaks, spills, or wrong storage. Digital checklists and team accountability take the guesswork out of storage routines, keeping rare but dangerous slip-ups at bay.
Moving Forward with Confidence
Responsibility goes beyond regulators. Everyone who opens a bottle of 1-cyanopropyl-2,3-dimethylimidazolium tetrafluoroborate steps into a chain of trust. Routines, not luck, stop accidents. If more people made safe storage their daily habit, chemical research would move forward on solid ground.
Why the Numbers Matter
Whenever working in a chemistry lab, those tiny decimal places by the element symbols pop up everywhere. They’re not just academic details — they're the key to calculations and real-life applications. Take a salt like 1-Cyanopropyl-2,3-Dimethylimidazolium Tetrafluoroborate. With its mouthful of a name, it shows up in discussions about ionic liquids, green chemistry, and next-gen electrolyte solutions. Anyone who’s built a molarity equation or weighed reactants knows: if your molecular weight is off, your experiment can fail or your data can get skewed fast.
Breaking Down the Molecule
No tool gives better clarity than the periodic table in front of your nose. For this molecule, things get a little elaborate, though. You’ve got the 1-cyanopropyl branch, two methyl groups attached to an imidazolium core, and the whole package balanced by a BF4- tetrafluoroborate anion.
Adding up each atom, with carbon at about 12.01, nitrogen at 14.01, hydrogen at 1.008, boron at 10.81, and fluorine at 18.998, you work your way through each group:
- 1-Cyanopropyl (C4H6N): C x 4, H x 6, N x 1
- 2,3-Dimethylimidazolium core (C5H8N2): C x 5, H x 8, N x 2
- Tetrafluoroborate (BF4): B x 1, F x 4
Piecing it together, you tally up:
- Total Carbon (C): 9 atoms
- Total Hydrogen (H): 14 atoms
- Total Nitrogen (N): 3 atoms
- Boron (B): 1 atom
- Fluorine (F): 4 atoms
A quick calculation: 9 x 12.01 + 14 x 1.008 + 3 x 14.01 + 1 x 10.81 + 4 x 18.998 brings you to about 288.30 g/mol. It’s a satisfying answer – and in my own years prepping solutions, double-checking these values has saved me money, reduced waste, and stopped unnecessary troubleshooting later on.
Importance in the Broader Picture
It’s easy to see numbers as just numbers, but take it deeper. Accurate molecular weights keep everyone honest on yields, purity, and even regulatory compliance. Chemicals like this get tested for toxicity, environmental safety, and more. Even a gram off the mark can skew research, especially in pharmaceuticals or materials science. The global push toward sustainable chemistry doesn’t work if basic data gets ignored — labs need trust in their ingredients, and trust only starts with correct molecular data.
Getting this one calculation right means fewer repeats in the lab and less anger at the end of a long day. Safety, efficiency, and scientific advancement all rely on nailing down basic building blocks of knowledge like this.
Spotlight on Solutions
Every chemist knows how easy it is to mistype a subscript or miss an atom tucked away in a name. Double-checking sources, using software, or collaborating on calculations in a lab notebook keeps accuracy in check. Teams who share reference databases or keep standard operating procedures up to date avoid most slip-ups. A company culture that encourages looking up molecular weights — not just relying on memory — winds up with fewer costly fixing runs.
One mistake with a sodium chloride solution might just need a little correction, but scale that to complex salts getting built into novel batteries or medicines, and the ripple effects reach the entire supply chain. The care put into something as small as molecular weight resonates far beyond the beaker.