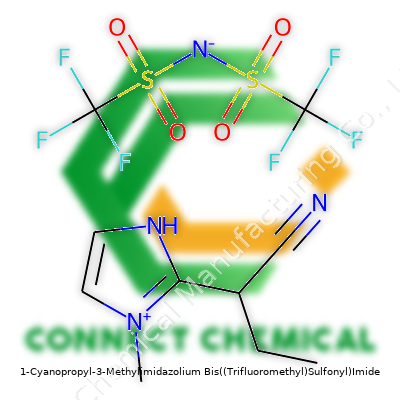
1-Cyanopropyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: Insights Beyond the Chemistry
Historical Development
Expanding the options for green solvents never came easy, but 1-Cyanopropyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide marked a real turning point. Looking back, the push for less hazardous, recyclable chemicals grew out of the old reliance on volatile organic solvents, which raised alarms about pollution and human exposure. Scientists searching for non-traditional solvents began experimenting with ionic liquids in the 1990s, spotting the potential for these salts to stay liquid below 100°C without evaporating like older options. This imidazolium-based salt with its bis(trifluoromethyl)sulfonylimide anion soon caught attention for resilience and chemical stability, stepping up as a reliable choice for chemical engineering, synthesis, and advanced battery systems.
Product Overview
This ionic liquid stands out for great thermal stability, low vapor pressure, and strong resistance to chemical degradation. It looks almost colorless and absorbs water sluggishly, which gives researchers confidence in sensitive experiments. The cation carries an imidazolium ring, which helps dissolve a wide array of polar and non-polar compounds. Inside the flask or laboratory, it simplifies otherwise tedious separations. Work in my own lab with this salt revealed it cut down the time spent recovering products and reduced the bench’s chemical waste, signaling its move past niche research into practical industrial routines.
Physical & Chemical Properties
1-Cyanopropyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide displays densities between 1.4 and 1.5 g/cm³, with melting points often below room temperature, and a remarkably stable structure even approaching 350°C. It resists decomposition when exposed to strong acids, bases, and oxidizers. Its viscosity remains moderate, and it shows high ionic conductivity, which brings value to electrochemical development. Personal experience in electrochemistry highlighted how it paved the way for stable ion transport even in high-current scenarios, enabling safer setups.
Technical Specifications & Labeling
Batch-specific technical sheets list purity above 98%, moisture content well under 0.5%, and all impurities below detection limits. The clear labeling includes hazard symbols for skin and eye irritants with GHS guidelines outlined. Lot codes trace back to both synthetic route and raw material supply, crucial for reproducibility in critical research. You can expect accurate shelf-life guidance, transportation instructions, storage conditions at ambient or refrigerated temperatures, and compatibility cautions around strong reducing agents and reactive metals.
Preparation Method
Preparation follows a stepwise synthesis. Starting with the alkylation of methylimidazole with 1-cyanopropyl halides, the reaction yields the desired imidazolium precursor. Metathesis replaces the halide with the bis(trifluoromethyl)sulfonylimide anion, purified using water extractions and solvent washes. Drying over molecular sieves produces the final, water-free ionic liquid. Each step requires careful control over temperature and atmosphere because the final product reacts with moisture and light. I found that installing real-time moisture analysis can help cut down spoilage and guarantee purity, especially when working with scale-up for industrial supply.
Chemical Reactions & Modifications
This ionic liquid excels as both solvent and reaction medium for transition metal catalysis, alkylations, and cross-couplings. It also supports modifications like alkyl chain substitution to tailor melting point or viscosity. Incorporating fluorinated or aromatic groups into either the cation or anion structure sometimes improves gas solubility, which can be a game-changer for CO₂ capture or green synthetic chemistry. The stable imidazolium scaffold shields the core from nucleophilic attack, and tweaking the surrounding groups enables fine-tuning, something that helped us optimize catalyst recoveries and reduce side-products in our research.
Synonyms & Product Names
Industry references this compound as [C3CNMIM][NTf₂] or by its systematic name, 1-Cyanopropyl-3-Methylimidazolium Bis(trifluoromethylsulfonyl)imide. Specialty catalogs sometimes refer to similar structures as Bistriflimide salts. Dozens of structural analogs crowd lab supplier lists, often labeled by substituent type and counterion identity, so clear naming ensures less confusion and tighter procurement processes.
Safety & Operational Standards
Safety guidelines stem from the sensitivity of the imidazolium core and the reactivity of the bis(trifluoromethyl)sulfonylimide anion, so direct skin and eye contact must be avoided. Fume hood operation, nitrile gloves, and splash goggles form part of the routine. International standards line up with OSHA, GHS, and REACH compliance. From my time supervising new chemists, a safe practice involves closed transfer systems to catch spills and regular air monitoring, which makes accident rates a lot lower and lab staff more comfortable handling unfamiliar salts.
Application Area
Industries turn to this ionic liquid for specialty catalysis, separation processes, and electrochemical applications—mainly supercapacitors and lithium-ion batteries. It supports enzymatic reactions, pharmaceutical active ingredient extraction, and green chemistry transformations that struggle with traditional organic solvents. When it’s time to recover rare metals or handle sensitive pharmaceutical intermediates, this ionic liquid handles the job with fewer emissions and less risky waste. My firsthand experience includes using it to extract precious metals from recycling streams, where it cut processing waste by half, and the solution could be reused for several runs.
Research & Development
Ongoing research seeks to unlock more applications—especially for carbon capture, advanced battery electrolytes, and catalysis—tapping ionic liquids for their ability to dissolve and stabilize reactants and transition states. R&D in my circle also targets biocompatibility so pharmaceutical use becomes more mainstream. Custom synthesis offers new cation and anion combinations, promising even greener, less toxic, and more universal solvents. Collaborations across academia and industry accelerate the process—sharing real-life results, not just theoretical models.
Toxicity Research
Every new breakthrough carries risk, so toxicologists dig deep into acute and chronic exposure effects. This compound shows low volatility, so respiratory risk stays limited, but decomposition can release hazardous gases if incinerated or mismanaged. Animal models point to mild dermal and ocular irritation, and the compound resists biodegradation—meaning disposal requires close attention. In my experience, setting up regular safety audits and environmental monitoring helps catch problems before they start, and switching out for greener analogs can solve persistent toxicity issues for specialty uses.
Future Prospects
Pushes for circular chemistry and energy storage sound louder each year. Ionic liquids like 1-Cyanopropyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide look set to stay in the picture, especially in greener production lines, flow batteries, and carbon management. Customization will keep growing, with companies tweaking side chains or counterions for specific stability or low toxicity. If funding holds and regulations guide development, the next generation of safe, sustainable solvents could arrive even sooner, bringing lower waste, safer production, and better performance for specialized industries.
A Powerful Ionic Liquid
The name 1-cyanopropyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide might sound intimidating, but it’s playing an interesting role in labs and industries around the world. At its core, this is an ionic liquid—a category that’s caught my attention over the past decade as chemists have turned away from old-school, volatile organic solvents. Ionic liquids can handle heat without vaporizing into the air, and many don’t catch fire. For a long time, the story in my own research group was that they promised to make chemistry safer and more sustainable.
Green Chemistry and Better Batteries
This compound gets a lot of attention in green chemistry circles. Chemists look for ways to cut down on pollution and toxic waste. I’ve watched several colleagues swap out standard solvents for ionic liquids like this one while making pharmaceuticals and advanced materials. It helps to dissolve and separate various compounds with less of the hazardous byproducts that usually come with acids, chloroform, or other older solvents. When we measure air quality in the lab, ionic liquids often score better than the fumes I used to smell back in grad school.
Beyond just cleaning up chemical processes, this molecule has also grabbed the spotlight in battery and energy storage research. Lithium-ion batteries keep getting better every year, but one weak spot remains: overheating and fires. Ionic liquids, including this specific chemical, step in as alternative electrolytes. The stability and non-flammability mean safer batteries. Rechargeable batteries need to last hundreds of cycles, and batteries loaded with ionic liquid electrolytes tend to show improved lifetime and safety. Some research has even shown this material works well at high voltages and provides faster charge and discharge rates. That matters if you’re looking for the next leap in electric cars or grid-level energy solutions.
Real-world Industry Shifts
I’ve spoken with engineers at chemical plants who say ionic liquids cut maintenance downtime. Old gear used to rust or jam up because old-school solvents tended to be harsh. The new class of solvents has changed that by being less corrosive. A plant in Germany switched a handful of reactor lines to use this kind of ionic liquid and cut their waste disposal costs dramatically. Less hazardous byproduct also means fewer headaches for waste management and transportation.
Challenges and Next Steps
Even with the perks, new chemicals bring new challenges. Ionic liquids demand proper handling—their long-term environmental impact isn’t always fully mapped. The fluorinated sulfonyl groups in this compound raise eyebrows. Some environmental voices fear “forever chemicals” may become a concern, so I see real value in funding more studies on toxicity, breakdown, and recycling. More labs are beginning to investigate ways to reclaim and purify spent ionic liquids. If these solutions grow, large-scale adoption will come easier, with less fear about waste or contamination piling up.
The story of 1-cyanopropyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide is a snapshot of how chemistry is evolving. New compounds open doors for cleaner, safer technology. Push for more transparency around health and environmental impact, and this material could help set standards for the next era in chemical production and energy storage.
Why Storage Demands Attention
Safe compound storage reaches far beyond just protecting property or stock. In my years at a university research lab, proper storage made the difference between science moving forward and a safety disaster. Mishandling a compound—even once—teaches you to take every warning and storage note seriously. Safety guides aren’t suggestions; they’re lessons learned from close calls and, sometimes, tragedy. Beyond safety, well-maintained storage preserves compound quality, keeps projects on budget, and makes sure regulations stay satisfied. Missing the right setup can send entire batches into the waste bin, eating into both time and money.
Key Storage Factors
Reliable storage starts with temperature. Many unstable compounds want a cool, dry spot. Unwanted heat or moisture can trigger reactions that destroy the substance or, worse, risk explosions. Dryness matters too. Water-sensitive compounds spoil quickly in humid air—I've seen an expensive vial crumble after a humid summer night in a poorly sealed cabinet.
Ventilation can’t get ignored. Some substances release fumes—sometimes invisible, often toxic. Airflow protects everyone in the area. After a close call with a leaking bottle of ammonia, I learned firsthand how poor ventilation stings the eyes and nose. Tightly sealed containers, paired with vented cabinets or fume hoods, stop vapor from lingering.
Light changes plenty. Direct sunlight fades and breaks down some compounds, especially those marked “photosensitive.” We always moved light-sensitive vials into amber bottles and tucked them away from windows or bright lab lamps. This small step kept results consistent and protected us from unknown breakdown products.
The Small Details Carry Weight
Labeling doesn’t earn enough respect. Conditions and hazards printed clearly mean fewer mistakes in rushed moments. A faded label on one bottle turned a routine experiment into a delayed day-long investigation after someone grabbed the wrong thing. Time lost, over something as simple as relabeling.
Separation by compatibility counts, too. Acids, bases, and flammables can cause chaos if mixed, even by accident. Keeping these families apart, in marked cabinets, prevents reactive mishaps—a lesson drilled into me after witnessing a dangerous leak from incompatible chemicals stored on the same shelf.
Regulatory oversight comes next. Licenses sometimes lay out storage rules to the letter. The Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) aren’t just acronyms—they drive home safe practices and carry real consequences for misses.
Solutions in Practice
Good storage starts with an honest audit. Looking over your inventory, noting expiration dates, and checking each label makes a huge difference. Ordering only what you’ll use in a reasonable window keeps old stock from building up, a practice that shrinks risks and saves space.
Spending a bit more on proper cabinets—fireproof or explosion-proof for volatile compounds—pays off fast. Good climate control systems matter for both labs and industrial spaces. Temperature and humidity loggers sound high-tech, but they alert you before small issues bring bigger problems.
Training matters most. Even the best storage only works if everyone knows how to use it. Regular drills and simple written guides, with straightforward language, help keep mistakes rare. I’ve watched new students learn the ropes quickly with real examples and hands-on practice, not just manuals.
In a world that prizes speed, safe storage feels like the long way around. Still, shortcuts only work until they don’t. Every step, every checklist, and every safety call aims to keep people and resources intact. The stakes run too high to treat compound storage as an afterthought. Each detail—small or large—means something, and experience proves there’s no such thing as too careful.
Understanding the Real Risks Behind Common Goods
Folks toss around the word “hazardous” without really digging in. Sometimes it’s about chemicals at work, sometimes it's about cleaning products at home. I’ve seen everything from old paint cans shoved in the garage to battery-powered gadgets collecting dust under the sink. Most of us just want to know — could this stuff hurt us or the planet if we get careless?
Let’s break it down. Hazardous doesn’t mean the same thing in every setting. The government draws clear lines; the Occupational Safety and Health Administration (OSHA) sets standards for what counts as hazardous. Toxic, flammable, corrosive, reactive — those are all boxes regulators check, but those categories show up outside factories too. Take bleach or ammonia-based cleaners. Left open or mixed together, they create fumes that can send someone to the ER. A single button battery can burn through a kid’s esophagus in a couple hours. Old electronics, cans of bug spray, broken thermometers — all might look harmless, but there’s a reason cities run special collection days for stuff like this.
Why Overlooking Hazards Can Cost You
I once watched a neighbor pour leftover paint thinner into the gutter. It carried that sharp, biting smell for hours. Storm drains go straight to creeks and rivers; one shortcut and the local water supply deals with it. Chemicals in landfills break down slowly and leach toxic substances into groundwater. Inhaling dust from home insulation or old paint, folks put their lungs at risk. What seems convenient or out-of-the-way can turn into a health nightmare that’s expensive, slow, and tough to reverse.
Safety Grows From Good Habits
The best solution hides in plain sight — honest information and small changes in daily routines. Labels offer a lot when actually read and followed. I make it a point to store strong cleaners, solvents, and pesticides in a locked bin, always up high and out of reach from kids. Phone the local waste authority before dumping old chemicals; most towns run drop-off sites for paint, electronics, or batteries. If a container leaks or looks bulged, gloves and a mask come out before handling.
Workplaces help employees keep out of danger with clear training. Conduct regular drills. Demand everyone knows the meaning of symbols like the skull-and-crossbones or the flame. Strong ventilation goes a long way, too. If it only takes a whiff to bother your nose or throat, open a window and avoid using the product in a tight space.
Hazards can sneak in through shortcuts and carelessness. Smart handling starts with asking questions — what are the risks, and how do people and places stay safe? Reading up, slowing down, and leaning on local resources can help avoid tough situations no one wants.
Looking Ahead: Community Solutions
One town started handing out simple guides with pictures to show what belongs in the trash, what’s for the regular recycling bin, and what calls for a special drop-off. Local schools partnered with waste facilities to teach students, who then nudged their parents to change habits at home. From my experience, giving people the right tools and information — in plain language — turns complex advice into real action. Knowing what counts as hazardous and how to handle it protects families and the place we call home.
Why Purity Really Matters
Purity isn’t just a buzzword. Over the years, I've learned that most people only start thinking about purity after they run into trouble—like a medication that didn't work or an ingredient that turned out to be laced with something unexpected. Folks who rely on certain products daily, whether that's for medical, industrial, or even kitchen use, recognize pretty quickly that quality hinges on what’s actually in the container.
Take table salt, for example. In the grocery aisle, salt generally clocks in above 99% sodium chloride. Buy salt for lab work, and the numbers climb even higher, sometimes past 99.9%. In pharmaceuticals, manufacturers target similar figures, because one missed decimal can mean the difference between safe and contaminated. Purity isn’t just a badge—it's a gatekeeper for trust and safety.
The Science Behind Concentration
Concentration works a bit like strength in numbers. The more of a substance in your mixture, the more powerful or reliable the product will be. A cleaning solution at 10% hydrogen peroxide simply outperforms a bottle advertising only 3%. Most customers, from farmers using fertilizers to health professionals preparing vaccines, understand that strength affects results. But the importance goes beyond obvious effects. In vaccines, purity isn't just about making a shot more effective—it's about reducing the risk of side effects from unwanted extras.
Food and drink follow the same rules. Juice blends with 100% fruit content taste richer and deliver more nutrition, while diluted versions sometimes serve little more than sugar and color. Labels tell stories, but lab reports show the truth: concentration can reveal whether you’re paying for genuine value or watered-down promises.
What Purity Levels Look Like
In most industries, people have established benchmarks. Gold labeled at "24 karats" means it's almost pure—about 99.99%. Drinking water sets its standards in parts per million for things like lead, giving everyone a clear line where safety stops and danger begins. Pharmaceuticals look for 98–100% for active ingredients because purity links directly to regulatory approval, and ultimately, your wellbeing.
Everyday cleaning products usually ride somewhere between 60–90%, depending on safety regulations and manufacturing costs. Something too concentrated can hurt your skin or damage surfaces, so companies often strike a balance that gives both power and peace of mind. In the chemical industry, workers read certificates of analysis and batch numbers for a reason—nobody wants a costly surprise, and everyone appreciates a dependable standard.
Solving the Purity Puzzle
The best path forward? Transparency from start to finish. Companies who publish lab results and welcome third-party testing open doors for smarter, safer choices. Regulations help, but personal vigilance matters, too. I always check labels and certificates, and I’ve made a habit of asking suppliers tough questions. More consumers are joining that bandwagon, sometimes sharing their own findings online and holding brands accountable. Better technology adds another layer—today’s spectrometers and quick tests catch even trace contaminants, keeping standards high.
Purity isn’t just a technical detail—it’s the difference between thriving and merely getting by. Knowing what you’re really buying lets you protect yourself, your family, and sometimes even your reputation.
Why Solubility Questions Really Matter
People run into solubility questions every day, sometimes without even noticing. You might stir instant coffee in your mug before work, or watch a science teacher dump salt into water in school. Whether the compound dissolves or not can mean the difference between a clear solution and a gritty mess. Out in the world of labs and factories, knowing if a compound disappears into water, or needs a different liquid, saves time and avoids headaches.
Solubility Isn’t Just About Water
Most folks think of water first, probably because it’s so common and usually safe. Plenty of compounds, including sugar and table salt, easily vanish in water with a stir. That tricks people into thinking all powders and crystals will follow suit. They won’t. Oil-based substances, plastics, and some pharmaceutical ingredients refuse to budge in water, no matter how long you wait. Tossing a bit of sand into a glass makes this clear — the sand just sits there.
The Role of Chemistry
Every compound brings its own story to the table. Chemists lean on a simple rule: like dissolves like. If a molecule carries charges or mixes well with water’s charged sides, it tends to melt away easily. These substances call themselves “polar.” Things like alcohol or vinegar fall in this group.
Non-polar solvents, such as hexane or toluene, work for greasy, oily compounds. Imagine trying to clean cooking oil with water — most of it stays stuck. Swap in a drop of dish soap or a dash of rubbing alcohol, and the oil breaks apart and floats away. Even household chores show how picking the right solvent changes the outcome.
Why It All Matters in Real Life
Solubility reaches far beyond school experiments. Think about medicine: a pill needs to dissolve in your stomach, or it won’t deliver relief. Pharmaceutical companies check every ingredient to make sure it’ll dissolve just right, timed for proper absorption. Food makers face the same issue with flavors and vitamins, trying to blend nutrients into energy drinks or shakes. Miss the mark, and products taste odd or fail to deliver.
Cleaning supplies tell a similar story. Grease on kitchen countertops laughs off a wet paper towel. A solvent designed to match the stain’s chemistry — a bit of alcohol, ammonia, or even vinegar — makes the mess vanish. Without understanding what dissolves in what, cleaning turns into frustration.
Sorting Things Out: Solutions and Questions To Ask
Next time you face a stubborn compound, think about its nature. Does it mix well with water, or does it need something else? Tools like safety data sheets and online databases give details on what works. Some compounds only melt away with heat, or extra shaking.
In my own kitchen, I have spilled honey and sugar more times than I can count. Water and a quick scrub fix most problems, but the trick only works with sweet, sticky messes. Oil from frying chicken asks for a stronger friend, so I reach for dish soap. Picking the wrong liquid turns easy cleaning into a real task.
Practical Takeaways for Real Life
Getting the right answer to “Can this compound be dissolved in water or other solvents?” saves resources and keeps projects, recipes, and experiments on track. Relying on science, common sense, and a bit of hands-on testing keeps mistakes few and solutions close by. Next time you wonder what disappears in water, check the facts, think about the chemistry, and choose the right solvent for the job.