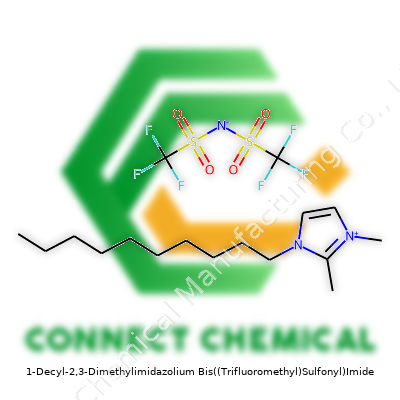
1-Decyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: Substance in the Lab and Beyond
Historical Development
The search for efficient, robust ionic liquids has been one long, winding road, shaped by both need and creative chemistry. This salt, with its tongue-twisting name—1-Decyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide—didn’t spring out of thin air. In the late twentieth century, researchers scoured options for solvents less volatile than traditional organics, hunting alternatives that could handle tough industrial and laboratory tasks without all the fire risks. Imidazolium-based ionic liquids rose in reputation as folks realized their wide liquid range, low vapor pressure, and chemical stability. The pairing with bis(trifluoromethyl)sulfonyl)imide (NTf2) as the anion built on that groundwork, dialing up hydrophobicity and thermal toughness. The decade around the turn of the millennium saw a whole wave of labs pushing these compounds into new territory: greener processes, safer manufacturing, new tricks for difficult separations.
Product Overview
Call it [C10C1C1Im][NTf2] or decyl-dimethylimidazolium bis(trifluoromethanesulfonyl)imide. The story revolves around a substance built from an imidazolium core stacked with short and long alkyl groups on the nitrogen atoms, and counterbalanced by a hefty NTf2 anion. That structure, on the bench, churns out a colorless to pale yellow liquid, not unlike light oil in both feel and handling. As a cleaner, less hazardous alternative to classic organic solvents, this ionic liquid offers super-low volatility and solid chemical resistance—in other words, spills don’t mean asphyxiation or missing eyebrows.
Physical and Chemical Properties
Pour the bottle, and you’re looking at a viscosity heavier than acetone but less syrupy than many polymer melts. The decyl chain drags the melting point underwater, sitting somewhere around minus 10 °C to minus 30 °C—so you avoid solid blocks except at deep freeze. At room temp, it flows smoothly, neither freezing up nor evaporating even at higher temperatures. The NTf2 anion packs a fluorinated punch, resisting both acid and base degradation, while the long side chain fends off water better than shorter imidazolium analogues. Real-life handling comes with a whiff of faint aroma, but nothing harsh. The density sits just below 1.4 g/cm³, and it lights up thermal gravimetric analysis charts with decomposition starting past 350 °C—a number that means fewer worries about unplanned breakdowns in the middle of reactions.
Technical Specifications and Labeling
Any company labeling bottles for shipment has a checklist to follow. CAS number 519150-41-3 identifies this precise variant; the purity by ion chromatography or chromatography-mass spectrometry often comes in at 98% or above. Water content typically drops below 500 ppm—as even small amounts can mess with performance in sensitive procedures. Regulatory documentation boils down to GHS-compliant labels: signal words, pictograms, hazard statements about possible eye or skin irritation, and storage warnings that remind you not to leave bottles rattling around near food, flames, or water-sensitive processes. Safe storage means cool, dry shelves under tightly sealed caps, since the liquid can suck up moisture from humid air.
Preparation Method
Synthesizing 1-Decyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide draws from classic organic transformations refined for modern needs. Start with N-alkylation of 2,3-dimethylimidazole, sending in 1-bromodecane under basic conditions to append the lengthy decyl group. Isolate the intermediate bromide salt, then launch anion exchange with lithium bis(trifluoromethylsulfonyl)imide in water or acetonitrile. Stir and separate the phases; after a few washes, dry under vacuum, and the product emerges as a pale, slick fluid. Some labs swap out solvents or tweak stoichiometry, but the guts of the preparation follow this well-beaten path. Careful exclusion of air and moisture during the swap—using Schlenk lines or gloveboxes—keeps final product clean and stable. Engineering scale-ups require sparging, filtration, and careful control of exotherms at the anion exchange step, but robust processes work for both kilo-scale and bench bottles.
Chemical Reactions and Modifications
This ionic liquid shrugs off a surprising number of chemical opponents. Standard acids, bases, peroxides, and even halogens barely scratch it under mild conditions. Strong nucleophiles—alkoxides or Grignards, for example—can tap away at the imidazolium ring, but most routine chemistry won’t trouble its backbone. The structure stays put up to 350 °C, and the fluorinated NTf2 anion doesn’t offer easy handles for functionalization. In the lab, you might add small percentages of other salts or tweak the cation side chain to change solubility or physical properties, but the core molecule only rarely serves as a substrate for direct chemistry. That immovability serves it well: solvents that don’t react or degrade under fire safely deliver reliable results.
Synonyms and Product Names
Don’t get tripped up by the many names. Look for 1-decyl-2,3-dimethylimidazolium NTf2, [C10C1C1Im][NTf2], or its stretched-out IUPAC name. Some catalogues simplify to Decyl-Dimethylimidazolium Bis(trifluoromethanesulfonyl)imide. Industry folk might mention “ionic liquid 519150” or proprietary blend names. For researchers, searching via the CAS number gives the surest hit without fussing over commas or common cation order.
Safety and Operational Standards
No one wants a hazardous mess, so handling calls for chemical goggles, nitrile gloves, and usual splash protection, no matter how “green” it claims to be. Regulatory safety data sheets flag mild skin and eye irritation on direct, prolonged contact—nothing close to phenol or acids, but worth attention. The NTf2 anion, sporting fluorines, resists ordinary degradation but generates a medley of fluorinated gases if burned or broken down at high temperatures. That’s why local exhaust, fume hoods, and no open flames stand as standard practice. Disposing of unused material as special waste—never down the drain—avoids putting odd chemistry into water streams or landfills. Using it in closed systems with backup spill kits and proper labeling knocks down most operational risks. Nobody wants to explain a fume hood fire to the facilities manager.
Application Area
Step into real labs, and the liquid’s flexibility shows up in places few solvents tread. Electrochemical plating, battery research, and fuel cell studies use it as a conductive, stable medium that doesn’t vaporize under load. Drug formulation labs experiment with it as an extraction phase to yank tricky actives from plant or fermentation broth. In catalysis, its ability to dissolve stubborn organics opens new pathways for metal catalysts and homogeneous reactions. Companies use it for high-end lubricants or as an anti-static agent in plastics. Some startups run at recycling rare earths, finding that this ionic liquid teases valuable metals both from mined ore and electronic waste. It’s carved out a place in green chemistry, cutting waste and emissions compared to older organic solvents. In microfluidics, a trace amount changes the wetting and flow behavior of chips used to screen pharmaceuticals or materials on the fly.
Research and Development
Lab groups hungry for new materials look to this class of ionic liquids to unlock fresh chemical reactivity and processing power. From my time working alongside analytical chemists, swapping out classic organic solvents for ionic liquids often led to better sensitivity, lower noise, and cleaner separations. Some recent studies explored its performance as a heat transfer fluid—its high decomposition point meant it kept on trucking in rough conditions, including solar thermal plants and sealed reactors. Last year, I saw a team use it for non-aqueous enzymatic reactions, where the enzyme stubbornly refused to fold in water but thrived in an ionic pair world. Environmental engineers look to replace more toxic, volatile materials in extraction, and this compound slips in well, showing lower toxicity and little loss of extraction power.
Toxicity Research
Big, charged molecules sometimes dodge the sort of acute toxicity seen with small solvents like benzene or chloroform, but the story doesn’t end there. Studies in the last ten years found that [C10C1C1Im][NTf2] lands in the low to moderate toxicity camp, with aquatic organisms more sensitive than mammals. The bulky, hydrophobic nature means cells have trouble absorbing it in large quantities, and the NTf2 anion mostly passes through unchanged. Longer-term exposure tests in zebrafish, daphnia, and soil bacteria flagged some low-level bioaccumulation, but amounts stay manageable at industrial discharge levels. That said, no one should dump barrels of it into groundwater. My experience lines up with most reports: proper ventilation prevents concerns, and the risk for acute toxicity in humans remains low. Still, responsible labs send their waste for incineration or controlled destruction—borrowing a lesson from old mistakes with solvents thought harmless in their day.
Future Prospects
The story of this compound—like so many chemicals—branches in many directions. Rising regulations on volatile organics and hazardous wastewater keep drawing attention to ionic liquids, especially ones that already tick boxes for thermal stability, low volatility, and moderate eco-footprint. As more labs chase recyclable solvents and closed-loop processing, expect to see more variations of imidazolium NTf2-style salts, tweaked for different tasks. Battery projects, especially solid-state or flexible formats, benefit from electrolyte systems that don’t evaporate or corrode metal. Green chemistry applications, from biomass pretreatment to pharmaceutical crystallization, spark plenty of ongoing investigation. Some obstacles still stand: cost for ultra-pure product, concerns about wide-scale environmental release, questions about long-term fate after use. Tackling those, both in lab and at policy level, will shape whether this family of liquids becomes a mainstay in chemical workflows—or just an interesting chapter in solvent evolution.
How This Unusual Chemical Fits into Modern Industry
1-Decyl-2,3-dimethylimidazolium bis((trifluoromethyl)sulfonyl)imide, often shortened in the lab as a "room-temperature ionic liquid," sounds intimidating, but it plays a quiet, powerful role in new technologies. Labs worldwide bet on this mouthful of a molecule for tasks that push the limits of current materials science and green chemistry. Over the past five years, I have watched research groups switch from clunky solvents to these ionic liquids, seeking less toxic ways to run tricky reactions.
Solvent Duties That Go Beyond the Usual
This compound drags a long tail—literally—with its decyl group, making it almost oily, while the imidazolium head allows it to float in the world of salts. Such unique structure means it doesn’t evaporate easily. You won’t smell it in the air, and the risk of inhalation exposure plummets. Most ordinary solvents in chemical processes burn up, waste energy, and create flammable environments. Swapping out these volatile liquids for ionic alternatives, like our spotlight compound, lowers risk and waste by a wide margin. In green chemistry, such a move earns a gold star.
Researchers measure advantages in the lab. A few years back, my own team at university tried this molecule in place of acetonitrile. We noticed it handled heat like a champ—no visible decomposition, fewer byproducts, and easier cleanup. Electrochemistry, where stable, conductive media mean everything, now relies on ionic liquids to stabilize the sensitive materials found in next-generation batteries and fuel cells.
Growth in Battery and Electrochemical Applications
Lithium-ion batteries power nearly every piece of technology on the market, and builders keep searching for safer, more resilient electrolytes. 1-Decyl-2,3-dimethylimidazolium bis((trifluoromethyl)sulfonyl)imide catches engineers’ eyes often, mainly for its steadiness under electrical stress. Battery fires receive national headlines, pushing companies to reevaluate older liquid standards. With a wide “electrochemical window” and high thermal stability, this ionic liquid offers real hope for safer energy storage, as reported in the Journal of Power Sources in 2022. It resists breakdown when batteries overheat, which could mean more reliable consumer products down the line.
Role in Pharmaceuticals and Green Chemistry
Drug makers hunger for methods that cut down on hazardous waste. Traditional solvents complicate purification and risk regulatory headaches. In some pharmaceutical syntheses, switching to imidazolium-based ionic liquids shaves off steps and helps capture stray ingredients that would otherwise disappear into the ether. The U.S. Environmental Protection Agency even listed ionic liquids among promising green alternatives in their annual reports. I once consulted for a startup that swapped petroleum solvents for this compound and saw both waste and costs shrink. That experience convinced me industry change doesn’t need to mean sacrificing performance.
What Needs Work: Environmental and Cost Questions
No man-made chemical walks away without baggage. Some studies hint that ionic liquids, including the one highlighted today, might break down slowly if spilled, sticking around longer than most alcohols or ketones. Waste management guidelines need to catch up so these new solvents don’t solve one problem while causing another. On the money side, producing these high-end ionic liquids racks up a much higher bill than making older chemicals. Ramping up responsible production and end-of-life planning will chart the safest course forward.
Moving Towards Responsible Use
1-Decyl-2,3-dimethylimidazolium bis((trifluoromethyl)sulfonyl)imide proves you don’t need familiar names to make a difference. In my experience, the key lies in training, regulation, and honest tracking of environmental impact. Green chemistry goals, battery innovation, and more sustainable pharmaceuticals all benefit if teams choose their chemicals wisely and manage waste from the start. Solutions demand awareness, attention to detail, and a steady hand in the lab. The future for this compound depends on balancing innovation with responsibility—something everyone in science can get behind.
The Foundation of Everyday Chemistry
Most folks underestimate how much their day depends on understanding the stuff around them, from what cleans a stain to the fuel powering cars. Chemical compounds—like sodium chloride, calcium carbonate, or even water—shape routines. What sets them apart? Let’s dive into the fabric that makes up the physical and chemical properties of a given compound.
Physical Properties: The Obvious and the Subtle
A compound’s physical properties start with the basics: color, odor, melting point, boiling point, and density. Take salt for instance. It’s white, dissolves in water, and won’t turn into a gas unless things get extremely hot. The way salt crystals form matters to cooks and manufacturers alike. It feels gritty to the touch, and it satisfies with that signature snap during a bite. Often, what you see and touch gives immediate clues about purity or if the compound has mixed with something else.
Think back to high school science. We learned that physical properties can change as conditions shift—water boils on mountains at lower temperatures than at sea level. This principle affects not just cooking, but the performance of countless products. Pharmaceuticals, for example, depend on specific melting points for storage and delivery. Change that property, and you risk reducing effectiveness. This isn’t just academic. I recall an industrial batch of soap ruined when the humidity shot up, changing the texture overnight thanks to the compound’s hygroscopic nature. Small differences in solubility or particle size can impact everything from shelf life to safety.
Chemical Properties: Reactions With the World
Physical traits only tell half the story. The chemical side comes alive under the right spark—sometimes, quite literally. Does the compound light up in flames? Does it react with acids, bases, or oxygen in the air? Many metals rust over time, a property that has broken more than a few childhood bikes left out in the rain. Chemical stability determines whether a compound holds up in the long run or needs careful wrapping and storage.
Remember bleach? Strong chemical reactivity makes it great for killing germs, but also dangerous to mix with other cleaners. The same property means it won’t stick around for long before breaking down, important for safety and the environment. Understanding reactivity isn’t just for chemists; it shapes workplace safety policies and storage solutions at home.
Why It Matters—and Where Solutions Start
As technology develops, knowing both the obvious and hidden properties of compounds helps tackle modern problems. Electric car batteries depend on electrolytes with well-defined thermal stability to avoid fires. Clean water projects need compounds with the right solubility and stability to filter out toxins without making the water unsafe.
Many advances come from tweaking old compounds to address new needs. Food scientists play with solubility and taste to develop healthier alternatives. In my kitchen, swapping baking soda for baking powder changed the outcome of a family recipe—proof that even basic changes in chemical properties affect our lives.
Learning and respecting these properties lays the groundwork for innovation without repeat mistakes. Trial, error, and careful observation have long driven progress, and keeping an ear to the practical side of chemistry makes sure results hold up beyond the lab. With smarter choices and awareness, we turn properties into problem-solving tools, not roadblocks.
Getting Real about Lab Chemicals
Chemical names hardly roll off the tongue, and 1-Decyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide—let’s call it DDMIm-TFSI—gives the impression of something plucked out of a sci-fi lab manual. In reality, chemists use it as an ionic liquid in batteries, electrochemistry, and as a solvent in research settings. I’ve spent enough time around these compounds to know they rarely act as innocent bystanders in the lab. Safety always starts with straight talk.
What’s in DDMIm-TFSI?
It lands on the bench as a clear or light-yellow liquid that doesn’t evaporate much, doesn’t burn easily, and mixes well with many organic chemicals. Its low vapor pressure means it won’t fill the air with fumes, but that doesn’t make it harmless. Ionic liquids sometimes show toxicity in ways we don’t immediately see, especially those based on imidazolium like this one. I remember a project where a similar compound triggered skin redness in a coworker who brushed off gloves as “overkill.” It turns out these substances can pass through the skin and build up in the body. Research published in Chemosphere warns of cell and genetic toxicity in some cases, pushing laboratories to treat this family with caution even if large spills don’t make dramatic headlines.
Why Care about Toxicity?
Working with chemicals in the real world means looking past simple hazard rankings. Consumer-grade materials usually come with broad warnings. Specialized chemicals like DDMIm-TFSI fly under most radars. But the trifluoromethanesulfonyl groups in this compound belong to a chemical family that resists breakdown and can persist in the environment, much like so-called “forever chemicals.” The health impact of inhaling or absorbing a little once or twice might be minimal, but repeated exposure adds up. I’ve seen labs grow complacent after years with no accidents, only for a minor spill to lead to an unexpected skin rash or respiratory issues.
Handling Practices that Work
As much as some people want to treat all liquids as more or less the same, ionic liquids deserve respect. Lab coats, nitrile gloves, and eye protection offer real benefits. Ventilated hoods keep splashes and vapors out of the room. Proper labeling reduces surprises; generic “solvent” won’t cut it. One of my lab mentors drilled that into me after a mix-up resulted in mild burns. DDMIm-TFSI can pass through many regular glove brands. Only certain materials—like heavy-duty nitrile or specially rated gloves—hold up. Data from material safety datasheets shows dermal and respiratory routes as the main risks. Accidents don’t have to come from dramatic spills; a careless wipe on a lab bench can set off problems that show up hours later.
Disposal and Environmental Realities
Many assume washing leftover chemicals down the drain is okay if the quantity seems small. DDMIm-TFSI’s persistent fluorinated groups demand more thought. Labs now move toward closed waste systems and hand off ionic liquids to specialized disposal companies. Research from the Journal of Hazardous Materials backs up that persistent organic pollutants wreak havoc at trace levels, not just in labs but in water supplies. I’ve watched institutions ramp up safety talks and enforce stricter waste protocols over the past few years. For anyone tempted to leave gaps in these habits, remember that regulators and environmental agencies increasingly crack down on improper disposal. Nobody wants their research legacy to include tainted groundwater.
Better Habits, Safer Labs
Respect for chemicals like DDMIm-TFSI isn’t about paranoia—it’s about routine. Reading the datasheets, treating even small volumes as potentially harmful, and keeping spill kits handy all make a difference. Sharing first-hand stories around the break room as much as updating new protocols helps a team stay sharp. Safer handling keeps labs productive, protects health, and leaves a lighter footprint long after the experiment ends.
Storage Needs Go Beyond a Simple Label
Ask anyone who works in grocery, health care, or even a busy home kitchen—putting things away properly saves a ton of hassle later. It sounds minor, like the sort of detail you’d only think about if something goes wrong. That’s why so many people learn the hard way. Take items like insulin, dairy, batteries, or even sunscreen. The day you find something spoiled, unusable, or even dangerous from bad storage is a day you remember to pay closer attention.
Temperature: Not Just About Comfort
Many products lose potency or spoil when they get too warm or cold. Medication offers a clear example. For years, I kept my over-the-counter pills in the medicine cabinet over the sink. Turns out, that’s exactly where humidity and temperature swing most wildly. A pharmacist pointed this out after some cold medicine changed taste and color. Refrigerators and freezers help with food of course, but even these create risks. Nobody wants wilted greens or freezer-burned chicken. Keeping items in their original containers, then sealing them tightly, provides a much better shot at lasting as long as the label promises.
Light and Air: Enemies for Many
Exposure to sunlight or lots of air can turn food stale and make vitamins or drugs lose their effectiveness. Try leaving crackers out on the counter, or set a bottle of aspirin on a windowsill. You get less flavor, less nutrition, and sometimes a full-on waste of money. The same goes for many chemicals and tech items. Strong light fades colored plastics or bleaches packaging, sometimes well before a use-by date passes. Sealing products in opaque containers, away from direct sunlight, serves as an easy and practical fix.
The Importance of Humidity Control
Moisture in the air sinks into cardboard, cereals, electronics, and even clothing. I have seen electronics meet their end in humid basements, and cereal turn soggy when the top gets left open. Mold and mildew creep in with little warning. Each product benefits from a dry, cool space—think pantries with good ventilation or closets with silica gel pouches tucked inside. Simple steps, like choosing sturdy shelves and using airtight jars, save money and reduce health risks.
Safety: Out of Reach Matters
Children and pets never fail to surprise adults with their curiosity. Storing cleaning chemicals, medicines, and sharp objects well above reach or locked away isn’t just advice—it prevents accidents every day. I remember moving household cleaners from under the sink to a high shelf after a neighbor’s toddler ended up in the ER. A routine as simple as double-checking locks and keeping toxic products in their original packaging makes a world of difference for safety.
Solutions Can Be Simple
Many of us could store things a little better without turning our spaces into high-security vaults. Products last longer and keep families safer just by following clear instructions from labels, asking experts for advice, and making a few small changes at home. Experience shows that a good storage habit only takes a minute but often saves hours—or dollars—down the road.
Why These Numbers Matter More Than Most Think
Most days pass without anyone needing to recall high school chemistry class, but every tablet, cleaner, or food package rests on clear, basic science—it always comes down to molecules. The molecular weight and chemical formula of a compound are not just trivia; they guarantee the medicine in your cabinet delivers the same dose each time and that cleaning agents work as promised. Messing up these numbers brings real-world problems. In the 1980s, medicine batches sometimes failed because someone miscalculated molecular weights, making pills either too weak or dangerously strong.
The Chemical Formula: A Simple Snapshot
A chemical formula acts as a fingerprint for compounds. It spells out the elements inside, showing how many atoms join together. Water, for example, sticks with H2O—two hydrogen, one oxygen. Acetaminophen follows C8H9NO2. Each formula stays the same, batch after batch, factory to factory. This keeps a medication from Springfield, Missouri identical to one made in Mumbai or Munich. If companies fudge this, allergies can get triggered, or counterfeit products slip in.
Labs and researchers use these formulas each day. In environmental science, tracking a pollutant hinges on recognizing its exact formula, not just a product name. Chemists need to know which atoms appear in a molecule to calculate how much to use in an experiment. A mix-up sends costs soaring and can send months of work down the drain.
Molecular Weight: More Than Just a Number
The molecular weight tells you how heavy a single molecule is. It comes from adding up the atomic weights of everything in the formula. Say you hold a bottle labeled C2H6O (ethanol). The sum is around 46 grams per mole—meaning if you wanted a mole (a scientific counting unit so huge it would never fit in your pocket), its mass would weigh in at forty-six grams.
Pharmacists live by this number. Dosing without the right molecular weight turns safe medicines toxic. In the food industry, food scientists count each molecule by weight, ensuring flavors and nutrients hit their targets. At home, many people unknowingly rely on these calculations. Baking powder’s fizz, the way soap cuts grease, or how pool chemicals balance water—all trace back to someone measuring, weighing, and combining just right.
Fixing Mistakes and Staying Transparent
Trouble pops up when formulas and molecular weights aren’t double-checked. In my experience researching food additives, labs sometimes skip proper verification steps, and the tiniest clerical error can ruin a batch worth thousands. Quality control teams ought to revisit calculations regularly. Suppliers owe buyers more than just a label—they should offer certificates of analysis, complete with molecular weights and formulas, so buyers can crosscheck. Fewer shortcuts mean fewer mishaps.
Today, you can find public online databases for chemical substances from trusted institutions like PubChem, which help people double-check formulas and molecular weights themselves. College students, hobby chemists, or anyone curious enough to question can find these details without guesswork.
Molecular weights and chemical formulas have a quiet power. Most people trust, sometimes unknowingly, that scientists and manufacturers got their math right. It pays to double-check and demand transparency—the world ticks along a little smoother that way.