1-Decyl-3-Methylimidazolium Acetate: A Down-to-Earth Review
Historical Development
The story of 1-Decyl-3-Methylimidazolium Acetate, or [DMIM][Ac], reflects the progress of ionic liquid science. Back in the 1990s, most chemists chased after “green solvents” to replace older volatile organics. Early alkylimidazolium salts such as [BMIM][Cl] got the ball rolling, but the need for tunable hydrophobicity and liquid range made scientists stretch the alkyl tails. Decyl chains entered the picture—turning old imidazolium salts into true chemical workhorses. As researchers fiddled with acetate anion, they found it opened doors for cellulose processing and deeper extraction work. Over the last two decades, this compound made the leap from academic curiosity to practical lab tool, showing up in projects from biochemistry to materials engineering.
Product Overview
1-Decyl-3-Methylimidazolium Acetate sits in the ionic liquids family. Its long, greasy decyl group teamed up with a methylimidazolium ring gives [DMIM][Ac] an oil-like touch. The acetate part pushes it into the hydrophilic territory but not so much that it dissolves everything like water. Workers in biomaterials turn to it for its ability to break apart lignin and cellulose, a job that clogs most solvents. In electrochemistry circles, it’s the smooth running carrier that refuses to evaporate or combust. Now, you’ll find it labeled with varying levels of purity but usually clocking higher than 95%. Storage needs a dry, cool spot, and that clear oil look signals you have the real deal.
Physical & Chemical Properties
At room temperature, [DMIM][Ac] looks like a pale to yellowish viscous liquid. Its long carbon chain boosts its density—often sitting at about 0.97 to 1.05 g/cm³. The stuff laughs in the face of water—it won’t boil until well above 200 °C. Its melting point sits comfortably below most room temperatures, making it practical for experimental setups without needing heat blocks or dry ice. Electrical conductivity sits a bit lower than smaller imidazolium salts, but it makes up for this with thermal stability. Solubility leans toward polar solvents, and the ionic set-up lets it work in organic and inorganic pools.
Technical Specifications & Labeling
Most full-spec labels list the compound as 1-Decyl-3-Methylimidazolium Acetate with a purity statement and water content—usually under 0.5%. Labels warn users to cap bottles tightly and store away from sunlight. Density, refractive index, and residual halide levels appear on better certificates of analysis. For research use, vendors pack in details about batch number, CAS number (1041387-41-2), and storage recommendations. Documentation points out compatibility with cellulose and notes on required PPE due to its amphiphilic structure.
Preparation Method
The lab route involves methylating imidazole first—then alkylating with decyl chloride to get the 1-Decyl-3-Methylimidazolium cation. After purification, workers neutralize the halide salt with sodium acetate, driving a metathesis that swaps out chloride for acetate. Control of moisture and oxygen levels matters through the whole process. Final purification usually pulls solvents off under reduced pressure, and people running large batches rely on liquid-liquid extractions and fine filtration. Mistakes at any stage can taint the ionic liquid with colors, halides, or left-over starting materials—so sharp analytical methods become part of the routine.
Chemical Reactions & Modifications
1-Decyl-3-Methylimidazolium Acetate reacts gently in most lab situations, but it holds some punch. As an ionic liquid, it holds special sway on phase-transfer catalysis, carrying ions between layers that neither water nor oil can bridge. On the acetate side, it works as a mild base. Heating it with certain organics can kick off acetylation, or in biorefining, it unpacks woody biomass by prying apart hydrogen bonds. Some research outfits swap the acetate anion for larger, non-coordinating anions to modulate physicochemical traits without rebuilding the cation scaffold. Researchers also try linking other functional groups to the decyl chain to further tailor properties for extraction, separation, or polymerization work.
Synonyms & Product Names
You might hear this stuff called 1-decyl-3-methylimidazolium ethanoate or DMIM Acetate. In catalogues, it hides under names like [DMIM][Ac], C10MIM Acetate, and simply Decyl MIM Acetate. CAS numbers and batch codes separate high-purity product from reagents designed for early-stage experiments. Labels sometimes list it as ionic liquid 17–1317 or 1-Decyl-3-Methylimidazolium OAc.
Safety & Operational Standards
Everyone handling [DMIM][Ac] needs to respect its skin and eye irritation risks. Gloves and goggles come standard, and ventilation remains a must—vapors carry organics and can sting the nose. Spills turn sticky—this stuff clings to benches and glassware, and cleanup suits best for absorbents and double-wash protocols. It doesn’t burn like ether or alcohol but slow, smoky decomposition at high temperatures puts out fumes you do not want to breathe. Waste streams can’t just hit the drain—ionic liquids get treated as specialty disposal owing to possible ecotoxicity. Labs that do it right confirm their local standards cover the byproducts.
Application Area
[DMIM][Ac] supports more than one field. Material scientists use it to spin cellulose nanofibers that get pressed into strong, lightweight composites. Biochemists dissolve and fractionate plant biomass, finding new ways to reclaim sugars and structural materials. In battery research, some groups mix it with lithium salts to boost ionic transport while reducing overheating risk in next-gen electrolytes. Chemical engineers find it helps them process and recover value from discarded plastics. In extraction chemistry, it pulls out tricky solutes while staying stable. The long decyl chain makes it a fit in surfactant and lubricant design for industries hunting new, less toxic formulations.
Research & Development
Academic and industrial labs keep [DMIM][Ac] on their shelves for pilot projects in sustainable manufacturing. European research initiatives focus on using it as a recyclable solvent for lignocellulose refiners. Teams in Japan and the US test its compatibility with emerging bioreactors and try to push its recyclability. Companies working with pharmaceuticals check how the ionic environment changes solubility and crystallization patterns for new drugs. The tuneability of the imidazolium core sparks curiosity among synthetic chemists—leading to tweaks that improve selective extraction, chiral separation, and even performance in CO₂ capture devices.
Toxicity Research
Toxicology studies flag up both low acute toxicity and some caution about long-term environmental build-up. The decyl chain makes [DMIM][Ac] less biodegradable compared to shorter alkyl analogues. Fish and water flea toxicity data show some chronic effects at elevated concentrations, especially in closed water systems. Mammalian studies usually report mild irritation but no carcinogenicity or acute hazards at amounts used in standard research. It’s not considered food safe and hasn’t cleared barriers for pharmaceutical excipients. Labs aiming at greener metrics continue to tweak side chains or pursue quick-and-total breakdown after use, aiming to sidestep future regulatory headaches.
Future Prospects
[DMIM][Ac] stands out in the green chemistry revolution. The demand for solvents that soften up tough biomaterials and allow for easier recycling keeps pushing research into functionalized ionic liquids with sturdy profiles. Regulatory pressure against legacy solvents puts more spotlight on compounds like this, encouraging manufacturers to invest in large-scale, high-purity production. Looking down the road, teams worldwide scan for less persistent, more degradable versions without losing the raw performance. Engineers want deeper tech transfer from bench to full-scale process—moving past small-batch academic demos to real-world impacts in troubleshooting waste management, renewable material synthesis, and safe, closed-loop chemical processing.
What Drives Interest in 1-Decyl-3-Methylimidazolium Acetate?
Walk into any lab working with materials science, green chemistry, or clean tech, and you hear talk about “ionic liquids.” 1-Decyl-3-methylimidazolium acetate belongs to that club. This compound has made waves because it breaks old limitations in how chemists process and transform stubborn materials, especially when those materials come from plants. Companies and researchers want tools that get more value out of waste—corn stalks, wood pulp, wheat straw—and this ionic liquid gets the job done where water or petroleum-based solvents fall short.
How It Assists in Making Biomass Useful
The world produces massive amounts of plant waste every year. Most of it gets burned or left to rot. The challenge? Lignocellulose—the combined bundle of cellulose, hemicellulose, and lignin—locks up useful sugars inside a tough matrix, making them very tricky to extract. With 1-Decyl-3-methylimidazolium acetate, that locked-up structure softens fast. Unlike harsh acids or expensive enzymes, this tool can dissolve cellulose into a liquid form, so scientists extract clean sugars for biofuels, biodegradable plastics, or specialty chemicals.
Why Choose This Compound Instead of Others?
Safety and performance both matter. Lab reports have shown that 1-Decyl-3-methylimidazolium acetate handles high temperatures and moisture without breaking down. That helps reduce dangerous emissions associated with older solvents. As an added advantage, this ionic liquid can often be recycled several times instead of thrown out after one use. That means less chemical waste heading to landfills.
Industrial Upsides and New Avenues for Research
Pulp and paper mills deal with piles of lignin-rich leftovers. In university projects, researchers use this compound to separate out high-purity cellulose fibers which can then be spun into new materials, like eco-friendly fabrics or strong films. Bio-based industries now look toward this extraction method to generate platform chemicals for pharmaceuticals and biodegradable cleaning agents. When farmers leave crop refuse in the field, it ties up resources that could drive rural economies. By harnessing new solvents, regional plants might one day turn this waste into profit, supporting communities while cleaning up supply chains.
Addressing Concerns and Looking for Next Steps
Relying on specialty chemicals raises important questions. Making ionic liquids at industrial scale takes both energy and raw ingredients, and the environmental benefit depends on responsible production. Some versions of these chemicals show toxicity toward aquatic life, which means the industry has to keep refining processes. Regulations push for thorough toxicity testing and safe recycling, which helps keep waterways clean. Engineers explore closed-loop systems so factories keep using the same batch again and again, limiting leaks or losses.
Practical Solutions: Collaboration Between Sectors
No one solves the waste or pollution challenge alone. Companies in textile, biofuel, and packaging sectors partner with academic research labs to test, adapt, and refine compounds like 1-Decyl-3-methylimidazolium acetate. Sharing data about long-term impacts will move things forward. I’ve seen firsthand how even small pilot projects unlock big change—less waste, new jobs, more sustainable materials. People need to communicate clearly about risks and rewards if these new solvents are going to shift the way industries work.
Chemical Formula and Makeup
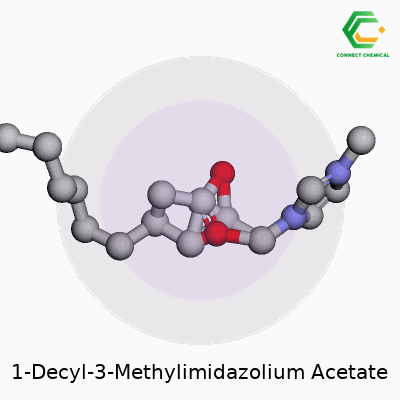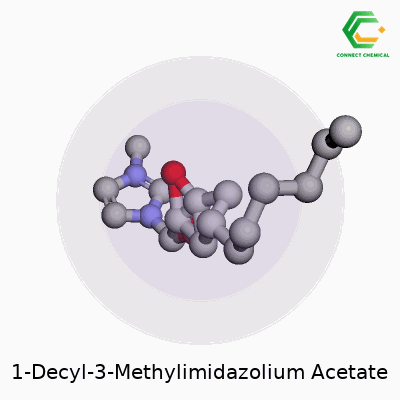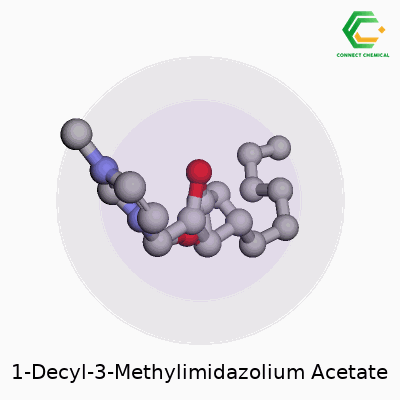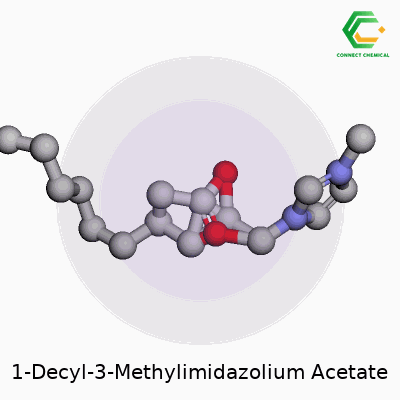
1-Decyl-3-methylimidazolium acetate offers a blend of a long alkyl chain and an imidazolium ring paired with an acetate anion. The formula stands as C16H30N2O2. Written out, the cation takes the form of 1-decyl-3-methylimidazolium: a five-membered aromatic ring with two nitrogen atoms, one methyl group on the third position, and a decyl chain (ten carbon atoms) on the first. The anion, acetate, sits as CH3COO-—a simple but robust partner for ionic liquids. These ions interact through electrostatic forces, setting the stage for the molecule’s unique properties.
Structure and Visualization
Structurally, the molecule stands out due to the long decyl group. That hydrocarbon tail injects flexibility, boosts hydrophobic character, and influences how the ionic liquid flows or interacts with organic compounds. On the imidazole ring, the nitrogen positions create strong ionic conductivity and hold onto thermal stability. A methyl substituent fine-tunes polarity. The acetate anion, recognizable in biochemistry, allows for hydrogen bonding, improves solubility in water, and plays a direct role in dissolving cellulose and other polysaccharides.
Pulling up a diagram, you’d spot a fused, flat ring with methyl and decyl sticking out, paired with a short, negatively-charged acetate right next to it. That structural layout affects both how the material behaves and how researchers use it.
Importance and Real-World Impact
This molecule makes a difference because it stands at the crossroads of sustainable chemistry. Ionic liquids like 1-decyl-3-methylimidazolium acetate sidestep the need for volatile organic solvents that often harm health and environment. Labs tap into its low vapor pressure, meaning harmful evaporations stay low. It handles stubborn biopolymers—think wood pulp or chitin—making it easier to create advanced materials or biofuels.
Researchers have seen real advancements using this compound for cellulose dissolution. Cellulose, the stuff plants make their cell walls from, doesn’t play nice in most solvents due to its tough hydrogen bonding. This ionic liquid can break that network without attacking the cellulose chain itself. That opens up greener processing routes for textiles and even medical hydrogels. In my time on a university team, we noticed instant improvements switching to imidazolium salts over traditional methods. Less toxic waste, easier recovery, and better product yield showed in every test batch.
On top of manufacturing, this class of ionic liquids enters the scene in electrochemistry and catalysis. The unique structure makes it possible to tune properties for specific needs—conductivity, viscosity, thermal stability—all without starting from scratch. As energy storage keeps rising in priority, safe and stable electrolytes like these look promising for batteries and capacitors.
Challenges and Forward Path
Not everything flows smoothly. Cost factors into widespread adoption. The synthesis route uses specialty imidazole rings and careful purification to avoid impurities, so prices stay high outside research labs. Many ionic liquids also linger in the environment and resist natural breakdown, raising concerns if scaled for industry.
Solutions lie in green chemistry. Researchers push for biodegradable substitutes that keep ionic liquid benefits without adding waste. Streamlining the production process reduces cost and brings this technology closer to everyday materials. More cross-disciplinary studies on toxicity and life-cycle impact help companies plan, not just react, making true sustainable adoption possible.
Overall, the story of 1-decyl-3-methylimidazolium acetate highlights how chemical structure links directly to performance and impact. Factoring in environmental safety, economic realities, and scientific need, this ionic liquid holds potential to unlock new materials and greener manufacturing—if users and innovators keep one eye on both the chemistry and the wider world.
Understanding Where 1-Decyl-3-Methylimidazolium Acetate Shows Up
People in labs know 1-decyl-3-methylimidazolium acetate mainly as a type of ionic liquid. This chemical dissolves cellulose, which opens up possibilities in textile processing, biofuels, and some greener-sounding solvent systems. It carries a reputation for replacing traditional, dirtier solvents, which at first might look like a clean win.
From what I’ve seen, chemicals that get billed as “green” don’t always live up to their marketing. Yes, they might cut down on smog-forming agents or nasty fumes. But every shortcut brings its own risks. So, this kind of ionic liquid needs some scrutiny before we hand out any awards.
Human Health: Reality Check in the Lab
Most studies about 1-decyl-3-methylimidazolium acetate point to a moderate to high toxicity in living cells. If you drop it on human skin, it can cause irritation. Breathing in the vapors or even getting a bit in your eyes spells trouble. In labs, nobody tests this stuff without gloves and a face mask. Some of my colleagues have handled it and reported a sharp, strong odor plus headaches after longer work sessions. You don’t want it splashing near anyone’s face.
Actual long-term studies in people are rare. Animal models show that these ionic liquids can accumulate in body tissues, especially with poor ventilation or sloppy use. That accumulation can throw off enzymes, disrupt cell signals, and might even screw up red blood cells. It’s not as deadly as old-school acids or lye, but it’s no picnic either.
Environmental Footprint: Tough Questions, Few Good Answers
There’s been talk that these liquids break down more easily in nature than fossil solvents. That’s only partly true. 1-decyl-3-methylimidazolium acetate breaks down very slowly under normal sunlight and bacteria action. Once out in the wild, this chemical lingers in water and soil. Aquatic species—especially tiny ones like daphnia and algae—suffer the most. Reports show it messes with their growth, can cause cell damage, and sometimes halts reproduction.
As wastewater treatment plants can’t completely filter it out, there’s a pipeline from factories or labs straight into rivers and lakes. The bigger the ionic liquid industry gets, the more we have to worry about these streams building up over time. Much like microplastics, nobody noticed their impact until it started compounding.
Staying Responsible: Safer Choices and Smarter Use
Safer chemistry demands more than switching out known hazards for lesser-known ones. Strong controls help: closed systems, glove boxes, rigorous waste management, real training for anyone who handles it. In my experience, replacing high-toxicity solvents with alternatives always calls for a full lifecycle review, not just a switch of ingredients.
Future research needs to look at how these chemicals break down in the real world and whether we can design even safer variants. Green chemistry wins by solving a problem without baking in a fresh set of headaches. In the meantime, organizations owe it to workers, communities, and the environment to treat 1-decyl-3-methylimidazolium acetate with the respect it deserves—never as a magical, harmless alternative.
Relying on Real Evidence, Not Hype
It’s tempting to get caught up in the promise of next-generation solvents. Still, cutting corners or taking marketing jargon at face value has burned the chemical industry before. For now, anyone considering broad use of 1-decyl-3-methylimidazolium acetate must look at both the upsides and the real, messy downsides. Keeping people and the planet safe always starts with honesty about the full picture.
Why This Chemical Calls for Respect and Prudence
Working with chemicals such as 1-Decyl-3-Methylimidazolium Acetate draws on everyday habits that echo through both labs and storage rooms. A bottle of the compound sits on a shelf or inside a cabinet, but there’s a lot more going on than quiet waiting. Based on my years in lab spaces, I’ve seen how chemical management often sets the tone for scientific trust and health. One mistake leads not just to spoiled material but to possible harm, clean-up headaches, and shaken confidence.
Protecting Product and People—Step by Step
A shelf by a window or a hot corner in the back room creates problems from carelessness. 1-Decyl-3-Methylimidazolium Acetate survives best in a cool, dry, well-ventilated spot. Direct sunlight can trigger chemical change, so avoid leaving the bottle in bright light. Humid environments encourage slow reactions with air and spoil purity.
Liquid chemicals like this can leak or spill. Tighten caps right after use. Store the bottle in a secondary container—one of those good plastic trays easily available in lab supply shops—to catch messes before they reach surfaces or drainpipes. Leaving the bottle on paper towels looks easy, but paper soaks up leaks and keeps danger hidden.
This chemical stings on skin and can irritate the eyes and lungs. Gloves—nitrile works well here—plus safety goggles keep contact to a minimum. Face masks with filters cut exposure to mist. Spend a few bucks and get a dedicated pair of lab shoes; solvent drops find socks. A basic chemical spill kit near the storage spot means any accident shrinks in scale, never growing into a serious incident.
Thinking About Labeling and Inventories
Labels matter. Strong, waterproof writing beats faded marker on old tape. Include the full chemical name, date opened, and a quick note about hazards. People often forget to check the bottle date, but fresh chemical acts differently than one left sitting for years. Toss old or degraded material. Running an inventory twice a year reduces waste, keeps surprises at bay, and matches regulations that local authorities are starting to require more often.
Room Conditions and Ventilation
This molecule sometimes releases small fumes, especially during transfers between bottles or mixes. Even a slightly sour whiff signals the need to boost airflow. Use the chemical inside a fume hood any time you pour, mix, or stir. Personal experience says labs feel safer—and smell fresher—when people refuse short-cuts on ventilation.
Disposal and Emergency Response
At some point, the bottle empties or the chemical goes off-spec. Dumping it down the sink is never safe and violates local rules. Put leftover material into the designated hazardous waste drum. Notify your facility manager about each disposal. Accidental splash on skin calls for fifteen minutes under cold water; don’t just wipe and move on. Report all spills and exposures, even if they seem minor—the small things often teach us the most.
Respect, Not Fear
Treating 1-Decyl-3-Methylimidazolium Acetate with care fits the daily routine of active, responsible research and industry work. Problems shrink when we put respect above haste, label carefully, and keep emergency plans visible and known to every person who shares the lab. Small changes in habit mean fewer close calls and stronger lab culture, something I’ve seen grow across decades of careful, diligent work.
Where to Find This Specialty Chemical
Anyone hunting for 1-Decyl-3-Methylimidazolium Acetate (often referred to in academic circles as [C10mim][OAc]) probably already knows their project isn’t basic high school chemistry. This ionic liquid sees use in dissolving cellulose, making for advances in biofuel research and material science. Most folks in research and industrial labs turn straight to specialty chemical suppliers rather than a general distributor. Major names on the scene include Sigma-Aldrich, TCI Chemicals, Alfa Aesar, and Santa Cruz Biotechnology. These suppliers keep inventories diverse for universities, government centers, and R&D divisions at private companies.
Online marketplaces such as VWR, Fisher Scientific, and specialized chemical shops offer direct catalog access. Most suppliers tell upfront if they can ship to your region and what handling requirements exist. Expect to answer a handful of due diligence questions, especially if the purchase goes to an address outside of a recognized institutional setting. For larger-scale orders, companies usually negotiate pricing and delivery through direct representative quotes.
Smaller, science-focused online retailers sometimes offer custom syntheses, but prices shoot up fast. They may be a better fit if a university lab or startup needs just a few grams and can’t hit the supplier’s usual minimum order. Students and young researchers often discover the challenge isn’t so much finding a website — it’s meeting the supplier’s verification procedures, which can block non-professional buyers.
Packing and Purity Information
A little experience in gel-phase chemistry or ionic liquids reveals the biggest concern when ordering isn’t just location or price: purity tells the real story. For 1-Decyl-3-Methylimidazolium Acetate, suppliers regularly advertise purities from 95% to 99%. Published research often calls for ≥98% for consistency in experimental results. Cheaper options at lower purities remain available, but even tiny traces of chloride or water will knock a project off track.
A standard product page often lists both “purity by NMR” and “water content by Karl Fischer titration.” Lab veterans learn to ask for both, since water mops up ionic liquids’ performance. For a cellulose-dissolving application, excess moisture causes cloudiness, unrepeatable data, and may straight out kill your day’s work. Some researchers even re-dry their ionic liquids in vacuum ovens before use.
Shipping regulations put certain constraints on this chemical. Most retail suppliers sell it in amber glass vials — common volumes include 1g, 5g, or 25g. Bulk material (like for pilot plant runs) needs proper drum labeling, often arranged by specialists in chemical logistics.
Staying Safe and Compliant
With any ionic liquid, safety protocols can’t take a backseat. Anyone making the purchase must read the supplier’s MSDS, check for alerts on skin or respiratory exposure, and prepare for any disposal issues. Some countries maintain import restrictions or documentation for specific ionic liquids (including acetate-based ones) because of rare but real environmental risks.
On the bright side, many larger suppliers certify their materials under ISO standards, listing not just chemical purity but batch traceability and shelf life. Verifying these credentials helps weed out counterfeits and “gray market” vendors — a lesson I’ve seen novice researchers learn the hard way.
Final Thoughts on Sourcing
Whether in research or production, buyers need to match supplier credentials, purity reporting, and handling to their end use. Speaking with technical reps or academic mentors often brings clarity, and reaching out early can dodge delays that slow a month’s work to a crawl. Purity impacts results, and the legwork in sourcing pays off every time an experiment runs clean.