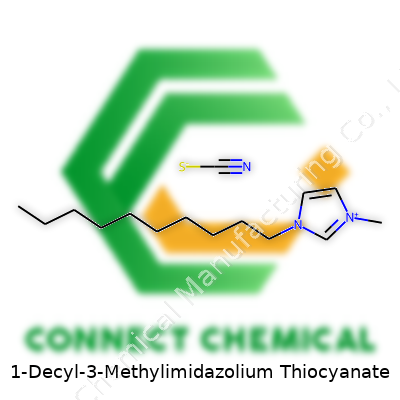
1-Decyl-3-Methylimidazolium Thiocyanate: An Extended Commentary
Historical Development
Curiosity around ionic liquids started to bubble up in research labs during the last few decades. Back in the 1990s, chemists realized these substances could unlock new paths for cleaner processes, energy storage, and advanced separations. 1-Decyl-3-methylimidazolium thiocyanate grew out of this surge of creative exploration. Researchers wanted an ionic liquid that stacked up well in terms of tunable properties, chemical stability, and ease of synthesis. With their quest for eco-friendly solvents in the background, these pioneers guided the design of compounds like this one, driving practical progress one experiment at a time.
Product Overview
This compound usually appears as a slippery, colorless or straw-yellow liquid. It embodies one imidazolium ring, a decyl chain, and a methyl group, all scored through with a thiocyanate anion. Thanks to its liquid state at room temperature, it fits a niche too tought to crack with older, volatile organic solvents. The combination of imidazolium cation and thiocyanate anion gives it high ionic conductivities and low vapor pressures, making it compatible with tasks spanning separation, catalysis, and electrochemistry. Manufacturers often target this ionic liquid for its blend of performance and relative ease of use compared to other tricky solvents.
Physical & Chemical Properties
Looking at its chemical backbone, 1-decyl-3-methylimidazolium thiocyanate stands out for being thermally stable up to about 300 °C. Its density typically lands around 0.97 g/cm³. Solubility varies: it dissolves easily in many polar solvents, from water to alcohols, and sometimes tolerates moderate mixing with nonpolar solvents due to that decyl tail. This ionic liquid barely evaporates, which matters a lot in workplace safety and long reaction runs. Conductivity clocks in higher than typical organic solvents because free ions move easily, which matters in fuel cells and advanced batteries. Viscosity depends on temperature, usually thick at low heat, more manageable once you warm it up a little.
Technical Specifications & Labeling
Sharpening up specifications, this product often arrives in glass or HDPE containers, with labels quoting purity (often over 97%), specific density, and water content. Safety info should always appear on the bottle or accompanying documents—signal words, hazard codes, batch or lot numbers to help trace quality. Many R&D units demand a tight spec sheet: low halide impurities, narrow water content range (well below 0.2% for sensitive processes), and minimal metal traces. Shelf life stretches longer than more reactive solvents if air and light stay out—sometimes topping a couple years if properly sealed.
Preparation Method
Typical syntheses of this ionic liquid kick off with an alkylation of 1-methylimidazole and 1-bromodecane, giving 1-decyl-3-methylimidazolium bromide. This intermediate then dives into metathesis with potassium thiocyanate. After a wash and drying, you end up with a high-purity liquid rich in decyl-imidazolium ions and free thiocyanate. Lab workers tend to lean on chromatographic or NMR techniques to check identity and purity. Scale-up brings its own hurdles—clean separation from byproducts and complete removal of starting salts generate the most headaches.
Chemical Reactions & Modifications
In research, new uses often grow out of tweaks to the imidazolium core or anion swap-outs. The decyl chain pushes solubility and viscosity in useful directions, hinting at custom blends for organic extractions. The thiocyanate anion can sometimes participate in S- or N-centered reactivity, allowing this liquid to act as a mild nucleophile or weak base. Crossover chemistry sometimes blends this ionic liquid with transition metal complexes or organic reagents, nudging selectivity in extraction, catalysis, or polymerization. Plenty of chemists build libraries by shifting the alkyl chain’s length or fiddling with the heterocycle.
Synonyms & Product Names
Search databases and you’ll find this chemical under a spread of names. Many labs record it as [C10mim][SCN], 1-Decyl-3-methylimidazolium isothiocyanate, or similar imidazolium thiocyanate terms. Some vendors use their own shorthand, but the critical part always marks the same two ions. For clarity, standard naming conventions usually spell out both cation and anion, cutting back on mix-ups when swapping data or ordering refills.
Safety & Operational Standards
Some see ionic liquids as “green,” but that label rings hollow if proper care drops out of the workflow. This particular compound can irritate eyes or skin after a splash. Gloves, goggles, and lab coats turn out to be minimum wardrobe near the bench or reactor. Ventilation should never get skipped—despite low volatility, the breakdown products from burned or spilled liquid could generate serious risks. Disposal requires a licensed outfit; dumping down the drain could land a lab in legal hot water, especially in regions with tight environmental rules. Each batch deserves a careful check of its SDS sheet, especially for users in innovative or scale-up settings.
Application Area
The useful jobs for 1-decyl-3-methylimidazolium thiocyanate have woven into several sectors—beyond just experimental chemistry. It sees action as a solvent for cellulose or polymer dissolution, in extraction of rare metal ions, and chasing after new cleaner catalysis methods. Electrochemists deploy it in electrolytes for batteries, supercapacitors, or even solar cells, thanks to conductivity and chemical inertia. Some recent papers report its value in carbon capture, especially when tailored for gas solubility. The push to phase out volatile organic compounds keeps nudging users in its direction, especially across universities and advanced manufacturing labs.
Research & Development
Much of today’s innovation circles around fine-tuning this molecule’s mix of stability, cost, and eco-friendliness. Big efforts target recycling the liquid after recovery, reducing contamination, or making greener anions that don’t hurt watersheds if a spill happens. Some startups aim to scale industrial processes using similar ionic liquids, but raw material cost, system integration, and waste recovery all challenge even the best project teams. Shared databases and journals keep refining best practices so new workflows can build smarter from the start.
Toxicity Research
Toxicologists haven’t closed the book on these ionic liquids. Acute and chronic effects in aquatic species or mammals sometimes come up around the anion’s mobility and subtle long-term risks. For this version, low volatility limits inhalation exposure, but skin contact or ingestion still deserve caution. Safety testing flags irritation, so regular risk assessments and exposure reviews matter for all users. Environmental persistence, breakdown products, and movement through wastewater systems invite further investigation, especially as labs and factories roll out bigger volumes. The promise of “green” chemistry sits side-by-side with the need to back claims up with real-world toxicology data.
Future Prospects
Looking forward, 1-decyl-3-methylimidazolium thiocyanate could unlock more applications if researchers solve its cost and end-of-life hurdles. Tying recovery and recycling into the workflow will help this and similar liquids escape niche uses and show up in broader markets, perhaps from energy to metals processing. Scientists now study ways to tweak both cation and anion, reduce toxicity, and extend safe use into industrial settings. Policies that reward sustainable chemicals or tax polluting solvents will drive adoption, but the science around lifecycle safety deserves constant attention. If the field continues growing, tomorrow’s ionic liquids may look back at this compound as a turning point on the road to safer, smarter chemical tools.
Unlocking the Use of an Unusual Ionic Liquid
Work in chemistry often circles back to solvents—those liquids that coax reactions along, clean up mixtures, or separate substances with quiet efficiency. Among the flashier modern solvents, ionic liquids have sparked interest for all kinds of reasons, but the workhorse 1-decyl-3-methylimidazolium thiocyanate stands out in a few unique ways. From my own time in the lab, I learned not all solvents pull their weight the same. Some float above the rest because they manage tough tasks with less fuss, which is what this compound tends to pull off.
Extracting Metal Ions: An Industrial Focus
People talk about “green chemistry” often right now, and ionic liquids like 1-decyl-3-methylimidazolium thiocyanate land in that conversation for good reason. It’s especially handy as an extractant—that is, it pulls certain metal ions out of complicated mixtures. Think mining, battery recycling, or cleaning up electronic waste. You want to tease copper, silver, or rare earth elements out of a tangled soup? This ionic liquid gets called in. The selectivity comes from its makeup: the thiocyanate part latches onto metals while the imidazolium wraps the whole affair in a stable embrace.
My first exposure to solvent extraction left me blinking at the separation process—parades of acids, lots of shaking—and a sticky mess at the end. Traditional solvents carry flammability risks and evaporate into the air. The world of ionic liquids flips that script. 1-Decyl-3-methylimidazolium thiocyanate hardly evaporates at room temperature. It has a stubbornly low vapor pressure, which keeps it in the flask, not in your lungs.
Cleaner Processing Means Less Pollution
Environmental agencies worldwide worry about hazardous waste. Solvent extraction with this kind of ionic liquid trims volatile organic compounds out of the picture. Not perfect, not a miracle, but a step in the right direction for safer handling and a smaller hit on air quality. In countries tightening their environmental rules, this property makes a big difference.
Labs and industrial plants alike appreciate a solvent that can be reused. 1-Decyl-3-methylimidazolium thiocyanate tends to hold up over multiple cycles. Fewer orders for refill barrels, less waste shipping, more cost control—details that matter once expenses start to stack up.
Challenges, with Room for Smarter Solutions
All the promise in the world doesn’t solve every issue overnight. The production of these ionic liquids still leans heavily on high-purity, sometimes pricey raw materials. Some remain toxic to aquatic systems if care slips and spills reach drains. Current research digs deep on recovery and recycling methods to re-capture ionic liquids from process streams. Smarter engineers and chemists keep poking at the edges, aiming for solvents that both do tough jobs and leave less of a mark when the work is done.
Looking Forward: Clean Chemistry Within Reach
Fact is, the world’s appetite for metals and electronics keeps growing. Old ways of extraction help fill that demand but bring steep costs in safety and pollution. 1-Decyl-3-methylimidazolium thiocyanate offers a glimpse of a future where selectivity, reusability, and low emissions matter more than brute force. Relying on facts from years of published studies, and the everyday patience of plant workers managing real-world problems, this ionic liquid proves its worth in places that need leaner, cleaner chemistry.
Real Risks Behind a Chemical Name
The moment I see a name like 1-Decyl-3-Methylimidazolium Thiocyanate, alarms ring. Chemical names this long usually belong to industrial compounds not found in your kitchen. Used mostly as an “ionic liquid,” it serves in research labs and sometimes in chemical manufacturing. Most people won’t run into it at home, but those working in science, engineering, or hazardous materials might deal with it up close. Knowing if it’s hazardous means looking behind the lab jargon and reading what real studies and safety sheets say.
What Science Tells Us About Toxicity
A lot of ionic liquids were developed to be “green” alternatives to solvents like acetone or chloroform. Some live up to the hype, but many, including certain imidazolium-based ones, have their own problems. Research papers collect stories of toxicity in aquatic species, cell lines, and, in rare cases, workers. When scientists in Europe tested several imidazolium ionic liquids, types with long alkyl chains—like decyl in this case—showed higher toxicity. The longer the chain, the greater the damage to fish, small crustaceans, and bacteria essential for wastewater cleaning. That’s a red flag, since thiocyanate itself can disrupt metabolic systems in plants and animals.
Lab animals exposed to similar substances developed liver problems or died at higher doses. Skin and eye irritation happened in lower doses, especially if the chemical wasn’t washed off right away. Most Material Safety Data Sheets for these chemicals carry warnings for skin and respiratory exposure, with recommendations for goggles, gloves, and even gas masks in small spaces.
Impact in the Workplace and Environment
From experience in a chemical research lab, workers treat these liquids with more respect than simple soap or bleach. The problem pops up if someone uses it without real ventilation or skips gloves for a quick task. Spills and improper disposal could damage local water systems since wastewater plants can’t always break down these chemicals. In cities and rural areas with weak water filtration, a small spill can move down pipes and rivers, harming aquatic life far beyond the lab.
Designer chemicals like this enter the market faster than regulations can keep up. Many countries don’t require full toxicity reports before researchers order them. That loophole puts pressure on users to educate themselves. So people in smaller or underfunded labs might not get the whole story before using the compound.
Making Safer Choices Moving Forward
The easiest way to shrink risk comes down to simple habits: read the safety data, use real protective gear, work with proper air flow, and double-bag waste. Dumping it into a regular drain could mean trouble. Some companies have started making “greener” imidazolium liquids with shorter chains or different ions. These seem to cause less harm in early studies, but switching chemicals costs money and takes time, especially for small businesses.
Waste management companies and environmental groups have begun sounding alarms on ionic liquids in water systems. They call for mandatory labeling, better training for workers, and real penalties for improper disposal. Sharing up-to-date risk data in plain language would help workers and communities. If labs and manufacturers share results about environmental effects openly, it prompts safer handling from day one. No single rule fixes everything, but better habits, clear communication, and stronger oversight make a big difference.
Understanding the Compound
1-Decyl-3-methylimidazolium thiocyanate doesn’t usually get tossed around in daily conversation, but for those working with ionic liquids, it’s a familiar face. The name itself gives clues about how the structure comes together. At its core, you have a bulky imidazolium ring — a five-membered ring of three carbons and two nitrogens — holding a methyl group and a lengthy decyl side chain. Tacked onto that is the counterion, thiocyanate, a molecule known for bridging the worlds of organic and inorganic chemistry.
Chemical Formula and Structure
The chemical formula for this compound looks like:
C14H27N2+ (1-decyl-3-methylimidazolium cation)SCN- (thiocyanate anion)
Put together, the complete formula becomes C14H27N2SCN, highlighting the separate parts without blurring the boundaries between cation and anion. The imidazolium ring keeps the molecule stable, even as the long decyl chain adds flexibility and tweaks the liquid’s properties. The thiocyanate anion, shaped like N≡C–S⁻, plays its own part, often impacting solubility and reactivity.
Why Structure Matters in the Real World
Knowing the formula and structure isn’t just for passing an exam or writing a label in the lab. I’ve met researchers who spend weeks adjusting the side chains on imidazolium salts like this, just to nudge their properties in the right direction. That decyl tail, for example, shifts the melting point and viscosity, making the liquid easier to handle at room temperature. Design decisions ripple through the process, whether it’s dissolving stubborn organic materials during recycling or soaking up charge in advanced batteries.
The thiocyanate anion can tip the scales, too. Swap it for something else — say, a halide or tetrafluoroborate — and the salt can jump between being water-soluble to barely mixing at all. This kind of flexibility means that scientists tailor ionic liquids for specific jobs. I remember one early career project, hunting for just the right combination so a liquid could strip heavy metals out of wastewater. The secret sauce? The length of the alkyl tail and the choice of anion, just as you see in this molecule.
Potential Solutions and Broader Impact
Ionic liquids based on 1-decyl-3-methylimidazolium thiocyanate open possibilities in green chemistry, especially for people who want to move away from volatile organic solvents. Unlike those solvents, these salts often show low vapor pressure, lowering inhalation risks in tight lab spaces. There’s also a marked shift in stability; the imidazolium core resists breakdown, making it viable across multiple cycles in chemical reactions.
Handling these chemicals calls for the same caution as with any reactive substance. Exposure to thiocyanate may affect health, particularly with chronic or high-level contact, pointing to the need for smart engineering controls and good lab habits. Investing in research to explore biodegradable or less toxic variations would pave safer ground. Many teams are already tweaking the structures, using natural feedstocks for the alkyl chains and developing greener anions, to shrink the environmental footprint.
Ultimately, understanding the structure isn’t just an academic pursuit; it drives safer, more effective solutions from the chemical bench to full-scale manufacturing, connecting the dots between molecular detail and real-world progress.
Kicking Off With What Matters
Keeping any lab chemical locked away and forgotten about rarely leads to good results. For 1-Decyl-3-Methylimidazolium Thiocyanate, storage takes a bit more intention. Tucked in a dry, cool corner, away from light and moisture, this ionic liquid avoids breakdown and contamination. Moisture in the air can turn even a stable salt into a sticky mess, making containers hard to handle and measurements unreliable.
Quality Counts in Every Detail
I’ve seen what happens in labs where flammable and reactive agents sit side by side, tempting fate with every passing shift. This imidazolium salt may not flare up like some startle-prone solvents, but it won’t win any medals for patience around oxidizers or strong acids either. It pays to give chemicals their own shelves—don’t squeeze this one beside nitric acid or even high-percentage bleach. One careless mix-up could spark toxic gases or even fire.
Easy Solutions in Organization
Labeling should never feel optional, especially with chemicals you don’t use every week. Printed, water-resistant labels listing chemical names, arrival dates, and hazard icons make inventory checks almost routine. Shelving chemicals alphabetically rarely works for anything but cataloging. For safety, sort stocks by hazard class, not just ease of reach.
Keep Storage Containers Up to Par
Plastic offers a false sense of security for storing many chemicals. I once watched a plastic cap soften and warp after holding an aggressive salt for six months. High-density polyethylene stands up to most ionic liquids, but always check the spec sheet: a leak turns a minor spill into a serious headache. For this compound, tightly sealed glass bottles keep things cleaner and more stable, locking out air and water vapor.
Access and Accountability
Limited access rooms are more than a bureaucratic hassle. They save lives. Only trained staff should grab for bottles containing sulfur, cyanide, or strong bases, even if they’re locked up. Most lab injuries start with someone grabbing the wrong stuff. Clear sign-out sheets and digital logs keep tabs on who took what, discouraging shortcuts and misuse.
Temperature—The Often-Overlooked Factor
Temperature swings wreak havoc on stability. Many ionic liquids drift into decomposition if left near radiators or sunny windows. Storing them between 15°C and 25°C avoids unexpected phase changes or breakdown. Fluctuating heat can warp even good caps, letting air seep in and start damaging reactions. If your storage room feels stuffy or drafty, it’s time to invest in better climate controls.
Emergency Preparedness Can't Wait
No one plans for an accident, but every lab expects one. Spill kits stocked with absorbent pads and chemical-neutralizing powders should stand within arm’s reach of storage cabinets. I’ve seen confusion slow down cleanups—no one remembers the protocol unless they’ve drilled for it.
Seeking Expert Advice
Consulting the safety data sheet (SDS) before storing any new chemical saves a lot of regret. These aren’t just forms for the compliance binder. They give single-page answers on incompatibilities, required ventilation, and first aid. A quick read at the start beats hours looking for answers in the middle of a spill.
Solid Habits Make Safer Spaces
Every step from labeling to regular temperature checks keeps the risk of storing 1-Decyl-3-Methylimidazolium Thiocyanate low. In my experience, the best labs keep a checklist handy and run through it at the end of every week. Staying vigilant keeps mistakes rare and work reliable.
Getting Familiar with the Chemical
Working around chemicals like 1-Decyl-3-Methylimidazolium Thiocyanate means stepping into a space that asks for attention and respect. In the lab world, I’ve seen how even basic things like labels and data sheets become game-changers. This particular compound falls into a family of ionic liquids now used in research and for specialized tasks like solvent extraction and catalysis. While it brings promise, no one should forget that safety beats curiosity every time.
Why Protective Gear Matters
Gloves, goggles, and coats form the everyday armor. I once caught a splash from a similar chemical on the back of my hand, and the burning itch served as a sharp reminder: bare skin is a recipe for trouble. Nitrile gloves usually give the best balance between flexibility and protection. Lab coats and safety goggles block most accidental sprays, and face shields can help if there’s a risk of bigger splashes.
Ventilation and Air Quality
Good airflow isn’t just about comfort. In poorly ventilated spaces, vapors or tiny droplets might linger. Fume hoods pull invisible threats away from your face and keep the air in check. My own workspace ran into false alarms in the past because a fume hood filter hadn’t been swapped out, and it led to headaches and nausea mid-task. It pays to double-check ventilation before uncapping a new substance.
Spills and Waste Disposal
During cleanup, speed can work against you unless you stay methodical. Spilled ionic liquids behave differently from water, and they can stick stubbornly to surfaces. Absorbent pads pick up most of it, but anything left behind gets cleaned using mild detergent and a lot of rinsing. Waste from this kind of compound never goes in the drain or a regular trash bin. Specialized disposal containers, marked clearly, give peace of mind not just for the current team but the ones who come next.
Health Hazards and Exposure Routes
Long names like 1-Decyl-3-Methylimidazolium Thiocyanate may mask their bite. This substance might irritate skin, eyes, and even the respiratory system if mishandled. Some data points to organ damage with repeated contact, which isn’t surprising given the trends across similar ionic liquids. Of the times coworkers forgot to fasten a mask or wore the wrong gloves, every incident drove home one truth: health risks don’t ask for your permission. Exposure often comes from splashes, dust, or inhaled vapors, and consistent use of basic gear limits the danger.
Training and Culture
The people who steer clear of trouble tend to be those who volunteer for routine safety training. Labs run better when newcomers get walked through emergency routines, like shower locations or eyewash stations. I learned early to memorize chemical numbers, but more keeps you safe—like proper transfer techniques and labeling every container.
Suggestions for Safer Practice
Labels that stand out and data sheets kept in a spot everyone can reach help cut down confusion. Maintaining a digital log of accidents and nearmisses shows trends others might miss. Stories help too. Sharing a mistake, like an incorrectly screwed cap, teaches more than a bullet list ever could.
Final Thoughts on Handling with Care
1-Decyl-3-Methylimidazolium Thiocyanate opens up new doors in science. Respecting its risks and keeping the focus on safety lets the benefits outweigh the hazards in any operation. Trust your instincts, refresh your training, and choose safety over shortcuts every time.