1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate: A Practical Look at an Influential Ionic Liquid
Historical Development
The story of 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate stretches back to breakthroughs in the 1990s, at a time when research into ionic liquids gained real traction. Chemists grew frustrated with the drawbacks of traditional organic solvents—flammability, volatility, and the risks these substances posed to both lab personnel and the environment. New ionic liquids emerged as alternatives, offering stability and low vapor pressure. As laboratories around the world sought less hazardous chemistry, this family of imidazolium-based salts captured the attention of both academia and industry. The development of 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate followed curiosity about how alkyl chain lengths and different anions might change the usefulness of ionic salts, much like tuning a recipe by swapping out its ingredients.
Product Overview
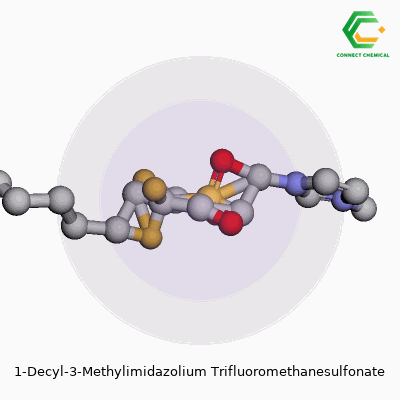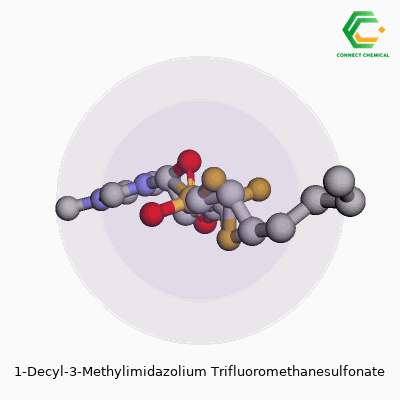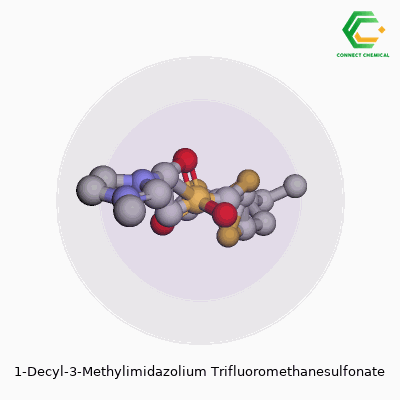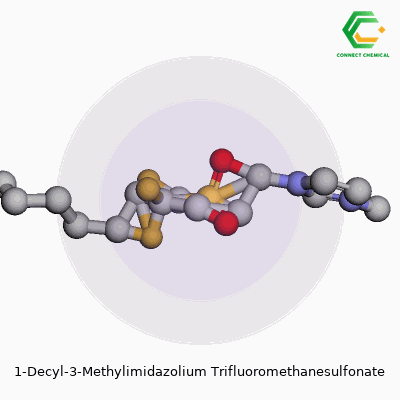
This ionic liquid, known more concisely as [C10mim][OTf], belongs to a group marked by their liquid state at or near room temperature. The pairing of a 1-decyl-3-methylimidazolium cation with a trifluoromethanesulfonate anion creates a combination prized for its stability, hydrophobic nature, and broad solvating power. Experienced chemists appreciate how the long decyl side chain can help separate phases in certain biphasic reactions and how the triflate anion tends to resist breaking down under tough conditions. Over the years, the substance picked up a list of aliases—1-decyl-3-methylimidazolium triflate, [C10mim][OTf], or sometimes just imidazolium triflate when the context is clear. No matter the label, it’s a staple for anyone working on advanced materials and experimental reaction media.
Physical & Chemical Properties
On the bench or in the bottle, 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate stands out with its clear, oil-like appearance. This liquid boasts a density hovering near 1.1 g/cm3 at 25°C. Its melting point sinks well below room temperature, a direct result of the twisted interactions between cation and anion. Volunteers in the lab find it doesn’t boil off quickly, even at elevated temperatures, and decomposes only above 300°C, giving it an edge in high-heat applications. This stability comes from the strong ionic interactions that bind components together, plus the extra stability delivered by the triflate’s electron-withdrawing trifluoromethyl group. It also carries a modest viscosity, flowing well enough for easy handling but sticky enough to dissolve or suspend heavy-metal salts, dyes, or catalysts. Its polar and non-polar regions let it interact with both kinds of molecules, a property that helps in extraction and catalysis.
Technical Specifications & Labeling
Producers usually list a purity of 98% or better, with water content below 0.05%, since even a little water can throw off results in sensitive reactions. Typical sample labels will include the C14H27F3N2O3S formula, molecular weight of around 372 g/mol, and identifiers like the CAS number 73899-12-8. For safe and precise laboratory storage, closed, moisture-free bottles, often inert inside nitrogen-purged cabinets, keep the ionic liquid in its best shape. For shipping over any distance, manufacturers have learned to pack the containers with extra care, as contamination from ambient moisture, plasticizers, or dust will occasionally disrupt the performance of the liquid in specialized experiments.
Preparation Method
Making 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate combines technique and patience. Synthesis usually starts with a quaternization step—reacting 1-methylimidazole with 1-decylbromide or 1-decylchloride to create the 1-decyl-3-methylimidazolium halide. After careful purification to remove side products, the next step is metathesis: mixing the halide salt with silver trifluoromethanesulfonate or sodium triflate. This switch replaces the halide with the triflate anion, and insoluble silver halide or sodium halide precipitates out, allowing for easy separation. Washing with organic solvents clears out byproducts, and filtration plus vacuum drying leaves a nearly pure ionic liquid. Getting consistent product depends on attention to detail; impurities from incomplete washing or improper temperature control can haunt later applications.
Chemical Reactions & Modifications
This compound shows striking resilience against hydrolysis, oxidation, and reduction. Chemists appreciate how the imidazolium ring remains stable even under basic or acidic conditions, while the long-side chain sticks out, useful for further derivatization or immobilization on solid supports. Reactions involving this ionic liquid run the gamut—phase-transfer catalysis, stabilization of nanoparticles, or dissolving biopolymers for novel material synthesis. In green chemistry circles, the non-reactive nature of the triflate allows it to serve simply as a medium, playing host for difficult transformations instead of participating in the chemical dance.
Synonyms & Product Names
Depending on the catalog and supplier, this ionic liquid appears as 1-Decyl-3-methylimidazolium trifluoromethanesulfonate, [C10mim][OTf], 1-decyl-3-methylimidazolium triflate, or even decylmethylimidazolium trifluoromethanesulfonate. Chemists searching scientific databases will find uneven naming, as conventions change with journal style guides and local preferences. Checking SDSs and labels before ordering has become second nature for researchers dealing with these nuanced differences. Despite the mix of names, once opened in a laboratory, the product’s odorless, colorless identity leaves no doubt—experience with the substance cuts through the confusion.
Safety & Operational Standards
Anyone who has spent time in a chemical lab knows that not all liquids play by the same rules. This ionic liquid avoids the flammability problems that haunt many traditional solvents, but lab workers still treat it with respect. Accidental skin contact should be cleaned up quickly, as the compound can irritate. Inhalation risks remain mostly low due to the liquid’s non-volatile nature; spills rarely fill the room with fumes. Waste disposal teams double-check for compatibility if the waste will mix with other chemicals, particularly because the fluorinated anion can linger in the environment. Labs reinforce basic safety: gloves, eye protection, and proper disposal bins, along with training protocols so even new interns handle such liquids responsibly.
Application Area
The fields that draw on this ionic liquid keep expanding. In my own work consulting on material science projects, its use in electrochemical devices, like batteries and supercapacitors, keeps increasing. Its stable, non-volatile profile makes it suitable for lubricants inside miniature motors, where precision and minimal maintenance matter. In pharmaceuticals, research teams use its solvating power to extract, separate, and purify drug precursors. Industrial chemical engineers exploring “green” solutions choose this solvent to run catalytic reactions that would burn through conventional organics. Academic groups push the liquid’s boundaries: they dissolve cellulose or chitin for biopolymer research or disperse metal nanoparticles for advanced catalysis. Each field latches onto this substance’s ability to deliver results that older solvents can’t.
Research & Development
The momentum behind research into [C10mim][OTf] shows no signs of slowing. Competitive labs and university teams focus on finding new uses—energy storage, rare earth element extraction, or biopolymer processing. Modifying the decyl group or the imidazolium ring, or swapping in other anions, sparks incremental discoveries in solvent behavior, hydrophobicity, and reaction selectivity. Large-scale applications face practical limits—the cost of raw materials, the energy used in synthesis, and the impact of scaling up—but ongoing development nudges these barriers downward. Cross-disciplinary collaborations enhance understanding, with chemists and engineers testing performance in live devices, rather than treating the liquid as just another bottle on a shelf.
Toxicity Research
Toxicologists and environmental scientists give ionic liquids close scrutiny. Early reviews suggested low volatility and decreased exposure, but deeper studies uncovered that long-term effects differ from one compound to the next. For [C10mim][OTf], moderate toxicity against aquatic organisms has emerged as a point of attention. Chronic exposure studies on human cells are less common, but short-term data indicate mild cytotoxic effects at high concentrations. Biodegradability remains a concern; compounds with long alkyl chains and fluorinated anions tend to resist breakdown in natural systems. These findings have already pushed some industrial users to tighten up containment, invest in better recycling protocols, and seek out less persistent alternatives. Real change takes time, but awareness fuels progress.
Future Prospects
With global attention focused on green chemistry and advanced materials, [C10mim][OTf] likely will evolve beyond a niche specialty. Improvements in synthesis aim to cut costs, boost yields, and lower the environmental toll. Regulatory bodies keep a watchful eye, and ongoing toxicity studies will guide policy decisions about widespread deployment. New variants—engineered cations, biodegradable anions, or tuned viscosities—keep cropping up, responding to shifting demands from electronics, pharmaceuticals, and renewable energy. My own view: the next generation of chemical engineers will treat ionic liquids like Swiss army knives—a versatile toolset requiring judgment, responsibility, and above all, a willingness to innovate and course-correct as the science deepens.
Chemical Formula and Structure
1-Decyl-3-methylimidazolium trifluoromethanesulfonate brings a mouthful of syllables, but it’s surprisingly straightforward at the molecular level. Its chemical formula reads as C15H29N2·CF3SO3H, which comes down to a cation-anion pair where the imidazolium ring wears a decyl and methyl group, balanced by a trifluoromethanesulfonate anion. Flourishing research in ionic liquids repeatedly circles back to this pairing of stability, solubility, and a knack for dissolving a vast range of organic and inorganic compounds.
Why This Chemical Matters
Lab benches everywhere seem to keep a tiny bottle of imidazolium salts, especially ones like this with a triflate anion. The reason for that stems not just from academic curiosity but also real-world needs — sustainable chemistry, better batteries, greener solvents. I remember the first time pouring out a small batch of this kind of ionic liquid during a university synthesis project. The substance had a viscous, almost oily feel, refusing to evaporate even after hours exposed to the air, quite different from the volatile chemicals I’d handled before.
By swapping out harsh organic solvents for ionic liquids such as 1-decyl-3-methylimidazolium trifluoromethanesulfonate, laboratories can cut health risks and waste. Triflate anions often deliver strong chemical stability and exceptional electrical conductivity, making the compound a natural fit for device engineers hunting improvements for supercapacitors or lithium-ion cells. Even pharmaceutical groups chase after these salts in their search for improved catalysts that avoid toxic byproducts.
Environmental and Safety Perspectives
This shift toward ionic liquids throws up new questions. Sustainability means more than swapping one liquid for another. To trust that an ionic liquid delivers on safety or greener chemistry takes more than a green label. Triflate-containing ionic liquids like this one often show low vapor pressure and lower flammability, certainly better for lab safety than acetone or diethyl ether. Still, their long environmental persistence brings up fresh concerns about water contamination, bioaccumulation, and the subtle risks that scientists puzzle over through toxicity studies.
The chemical world often rushes to adopt new tools before their full environmental stories get written. We can look at the way perfluorinated compounds stuck around in the environment long after initial optimism wore off. The fluorinated part of the triflate anion means chemists have to treat waste with caution, investing in appropriate disposal systems to avoid the mistakes of the past.
Steps Toward Smarter Chemistry
As people start to use more of these ionic liquids, there are a few lessons to carry forward. Monitoring and regulating lab waste, for starters, should be standard. Academic labs and chemical manufacturers alike ought to publish complete environmental profiles for any new ionic liquid they put out. That would mean disclosing toxicity data, degradation rates, and actual environmental impacts.
Researchers should also spend time developing recovery and recycling techniques for 1-decyl-3-methylimidazolium trifluoromethanesulfonate. Closed-loop processes — where solvents are purified and reused — protect both the wallet and the world outside the lab. Plenty could change with government incentives for greener solvent technology, rewarding researchers building safer, less persistent ionic liquid families.
A well-designed chemical formula says a lot about our priorities as scientists and citizens. By treating compounds like 1-decyl-3-methylimidazolium trifluoromethanesulfonate as part of a bigger environmental system, chemists keep progress from coming at too high a cost.
Everyday Workhorse: Daily Needs and Convenience
Think about the last time daily life called for a bit more convenience or reliability. This product steps up in small but significant ways. I’ve pulled it out countless times for tasks most folks don’t even notice happening — fixing leaks around the home, sealing up containers, or patching up surfaces that take a beating. In the workplace or around the house, dependable materials matter. Without them, simple fixes might stretch into hours of hassle or lead to bigger, more expensive repairs down the line.
Across neighborhoods, professionals count on this product for quick repairs because it’s easy to handle, keeps messes to a minimum, and rarely lets anybody down. Construction crews, for example, turn to it because they know it holds fast, even under stress or shifting temperatures. Day-to-day, it’s the behind-the-scenes champion that lets us keep going about life with fewer interruptions.
Healthcare and Medical Applications: Trust Built Over Time
Hospitals and clinics require tools that do the job right the first time. No one wants uncertainty during medical care. Doctors and nurses trust this product for securing bandages, supporting wound care, and helping keep equipment in place during important procedures. Even for small tasks during recovery at home—covering minor cuts, managing medication schedules, or protecting sensitive electronics—it continues to prove its value.
This level of trust only comes with proven safety standards and ongoing oversight from regulatory agencies. Existing research supports its role in medical environments, showing low rates of allergic reactions and reliable hypoallergenic properties. I’ve seen caregivers breathe easier with tools that don’t cause more problems than they solve. It’s clear that behind every routine application is a history of trust and accountability.
Industrial Power: Keeping Work Moving
Manufacturing rarely slows down, and reliable materials keep the gears turning. This product pulls double duty on assembly lines—sealing, binding, or keeping surfaces protected from grit or moisture. Factory workers appreciate durable solutions that reduce the risk of defects or downtime. Aerospace and automotive industries also lean on tough, adaptable products during critical production runs. In these environments, nobody wants to gamble on quality because even a minor failure can mean bigger costs or safety concerns later on.
Statistics from sector surveys show a consistent uptick in demand over recent years. Companies prize this particular product for its versatility and cost-effectiveness. I’ve spoken to maintenance managers who stress the importance of minimizing downtime and improving worker efficiency, both of which hinge on reliable supplies.
Education, DIY, and Creative Uses
Classrooms and home hobbyists also find plenty of ways to use this product. Teachers use it for art projects, quick repairs, and even science experiments. Whenever kids dive into crafting or model-making, a strong, non-toxic supply makes all the difference. Safe, easy-to-use materials mean less worry about accidents and wasted time.
DIY enthusiasts and artists appreciate this product’s flexibility. Creative minds tackle furniture repairs, organize cables, or add finishing touches to personalized gifts—all using this trusty staple. Social media channels are full of inventive project ideas, sharing inspiration and proving that a simple tool can spark a whole lot of creativity.
Looking Ahead: Improving Access and Safety
Strong demand puts pressure on manufacturers to keep refining the formula while meeting safety and environmental standards. Sustainable sourcing and ingredient transparency have become growing concerns for both businesses and households. Everyone wants confidence in the products they bring home or into the workplace. Open conversation between companies, regulators, and consumers helps drive innovation while reducing worries about toxic ingredients or waste.
With real-world solutions, practical know-how, and steady oversight, this product continues earning its place in toolboxes, medical kits, and classrooms around the world. Whether you’re patching up a pipe, fixing a project, or helping someone heal, it quietly helps keep life moving in the right direction.
The Risk Behind the Name
It’s not every day that you come across a mouthful like 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate. For most folks in labs, working with chemicals this complex has become second nature. But experience has taught me—no matter how familiar a name starts to sound, the risks don’t get any smaller. This ionic liquid brings plenty of promise, from solvents in chemical synthesis to electrolytes in special batteries. Still, the need for careful handling stays front and center.
Personal Protection Starts with You
I remember the first time I handled an unfamiliar ionic liquid. A glove ripped in the middle of a transfer, and I realized, gloves aren’t suggestions—they’re the difference between a regular day and a trip to the health services office. Nitrile gloves handle this task well. Chemical splash goggles keep eyes safe from sneaky droplets that never announce themselves. A lab coat doesn’t just keep spills off your clothes—it buys you a second to react when something splashes unexpectedly.
Respecting Fumes, Not Just Liquids
Some colleagues shrug off proper ventilation as overkill, especially for chemicals that don’t smell strong. Ionic liquids sometimes fly under the radar because of low vapor pressure. Even then, it doesn’t mean fumes won’t irritate lungs or quietly attack sensitive tissues. Always pour and transfer inside a fume hood. I once thought a room’s open window worked just as well—until a friend with asthma showed what a difference a fume hood makes after just a minor spill. Open windows can’t replace forced ventilation designed for chemistry work.
Cleanups: Quick Action, No Shortcuts
Spills aren’t rare. Quick response makes all the difference. I learned early to keep spill kits close—absorbent pads at the ready, gloves within arm’s reach. Soak up the liquid first, then sweep with a dedicated dustpan. Always wash the area with water and a bit of detergent, since invisible residue has a way of showing up on chromatography results even after a “good enough” cleanup.
Storage: Out of Sight, Out of Mind Isn’t Enough
Clear labeling makes storage much simpler. I’ve seen more than one accident blamed on “I thought it was just acetone.” This compound needs a secure, cool, dry spot, away from acids, bases, and anything that can react with organic solvents. Locks deter wandering hands, especially in busy labs with lots of shared space. Never leave open containers—moisture from air changes the properties of ionic liquids in a hurry.
Waste Disposal Demands Honesty
Some folks think a tiny chemical waste pour-down-the-drain won’t hurt. The truth: a single mistake could shut down your lab and lead to heavy penalties, not to mention harm to public waterways. Always dispose of spent 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate in labeled hazardous waste containers. The university environmental health office once sent out a stern letter after someone ignored protocol. It cost the department two days of lost work and a lot of trust from upper management.
Training Is the Best Shield
No checklist replaces hands-on training. I owe my best habits to seasoned lab managers who drilled safety rules into every new student. The National Institutes of Health recommends annual refresher courses, but practice every shift keeps safety sharp. Ask questions when you’re not sure. You’ll save yourself—and your labmates—a lot of trouble.
Building the Culture
In lab work, trust grows through shared habits and open reminders. I’ve learned nobody minds if you remind them to check gloves or double-bag their waste. The safest labs aren’t those with the fanciest safety gear. They’re the ones where people look out for each other and stay honest about the risks, every time.
Safer Storage: Consistency Counts
Working with chemicals like 1-Decyl-3-Methylimidazolium Trifluoromethanesulfonate demands respect, not just for its reactivity, but for the people and research budgets relying on its stability. Keeping the bottle on a random bench only invites headaches down the road. I’ve seen finely-tuned projects go sideways because of sloppy storage choices. Over the years, one principle has held: controlled temperature makes a difference. Most ionic liquids reward you with longer shelf life if you stash them around room temperature, out of direct sun, and away from heat sources. Excess humidity or wild temperature swings—coming from careless warehouse setups or cracked HVAC—speed up degradation and introduce moisture inside the bottle.
Secure Containers: Don’t Cheap Out
A sturdy, tightly sealed glass container isn’t optional. Those plastic lids you find on generic lab bottles may turn brittle or react with aggressive salts, especially if a bottle has been around longer than you think. I used to work in a place with loose lids, and after one warm weekend, we returned to sticky residue and a far bigger cleanup than anyone had expected. Thick-walled glass, with a new cap and a clear label—these choices save money and product. Make sure the container says what’s inside, who opened it, and when.
Keep It Dry: Watch That Humidity
Moisture sneaks in through cracked seals and careless hands. This salt has a habit of soaking up water from the air. Once moisture gets in, purity drops and reproducibility vanishes. I once tested some recovered samples after students stored them in a high-humidity stockroom. Even a few days changed their viscosity and color. If you must open the vial, do it in a dry box or quickly swap out what you need. Desiccators work—choose a chemical desiccant and replace it before it’s spent. If left in a regular fridge, the risk of condensation rises, so make sure it’s sealed before cold storage.
Avoid Contamination: Think Ahead
Cross-contamination creeps up through sloppy use. Designate specific glassware and spatulas for this chemical, especially if the lab works with metals or acids. I believe labeling and consistent habits shape a culture of safety. Once, someone dipped a damp spatula into our ionic liquid stock. The next project couldn’t get the same results as before, which cost weeks of repeating tests and sourcing new product. A chain is only as strong as its weakest link—attention to detail matters.
Plan for Waste and Spills
Even the best routines can’t prevent every accident. Have a waste container handy, labeled for this chemical. A spill tray or mat around the storage area catches drips before they become a bigger mess. I make a habit of asking new colleagues about their spill routine—not because I expect disaster, but because habits shape outcomes. If anything spills or leaks, scoop it with dedicated absorbent and dispose of it per hazardous waste rules.
Quality Storage, Quality Results
Every experiment starts with the chemicals you trust. If storage suffers, purity slips and experiments falter. Staff training, reliable gear, a good record-keeping habit—these choices protect everyone’s time and safety. I’ve saved myself countless headaches by respecting storage basics, and I encourage colleagues to do the same with every jar or bottle that comes through the door.
Why Solubility Matters in the Real World
Ask any chemist—or anyone working in healthcare, agriculture, cleaning, or food science—about solubility, and you’ll likely hear stories about ruined batches and wasted materials. Solubility means how well a substance dissolves in water (or another solvent) to form a solution. This isn’t just jargon for lab folks. If you mix a painkiller into water at home, dissolve fertilizer into a watering can, or stir sugar into your coffee, you’re relying on solubility. It touches medicine, farming, cleaning products, and even your glass of iced tea.
What Impacts Solubility
Chemists look at a substance’s structure—the type of atoms, the shape of the molecule, and how they all stick together. Charge matters. Table salt, for instance, quickly disappears in water. Oil, on the other hand, floats and swirls. Temperature and pH shift things as well. As anyone who’s tried to mix powdered cocoa into cold milk can tell you, heat often gets molecules moving and breaking apart. Some solvents work better for certain materials. Nail polish remover (acetone) attacks ink, adhesives, and, of course, nail polish, but leave it near your vinyl flooring and you’ll see the true meaning of “aggressive solvent.”
It’s Not Just Science—It’s Safety and Performance
Solubility can carry real risks and benefits. In pharmaceuticals, if a drug doesn’t dissolve properly, it may never reach the bloodstream the right way. That can turn a life-saving pill into something useless. In cleaning, the wrong mix leads to streaks or leftover grime. For farmers, improper mixing affects how well a pesticide spreads, possibly harming crops or wasting money.
According to the FDA, most oral medicines must dissolve in water before they work in your body. Drugs with poor solubility often need extra help—special coatings, new formulations, or added chemicals to help them break down after swallowing. Regulatory agencies don’t just suggest best practices; they require evidence of dissolution in banks of stability data.
Testing Ways Most People Don’t See
Many companies skip lab checks and lean on supplier data. That approach saves a few bucks at the start but can cost millions if complaints flood in or a product recall happens. Running a simple shake test or investing in a small-scale dissolution simulator tells a story no printed brochure ever will. For those in the know, a clear solution (or the lack of one) means more than a thousand words.
What We Can Do Better
A big step forward would be making solubility data public and updating it for real-world conditions. Research doesn’t trickle down fast, so companies and even consumers face gaps. Transparent labeling about what dissolves and what lingers helps everyone—not just big labs. Companies can list compatible solvents directly on packaging, cutting down on confusion and waste.
New education tools bridge the gap between the lab and your kitchen sink or garden hose. If folks knew why soap scum clings to tubs or why powdered supplements clump in drinks, they could make better choices. Knowing a product’s real dissolve rate under normal conditions is as crucial as knowing its shelf life.
Real-World Solutions Rely on Shared Knowledge
Factories, research labs, and homes all run smoother when folks can trust the information on a label or website. Solubility, once just a detail for chemists, now lands squarely in the hands of anyone trying to make, grow, clean, or heal. It’s not just about what dissolves, but about making sure your next project or prescription works exactly as expected.