1-Ethoxyethyl-3-Methylimidazolium Bromide: A Deep Dive
Historical Development
Curiosity around ionic liquids like 1-ethoxyethyl-3-methylimidazolium bromide started growing in the late 20th century. Chemistry labs in the ‘90s felt excitement about the possibilities these salts could offer as solvents that skip the problems caused by volatile organic compounds. The rush for greener, safer chemical processes turned attention to organic cations paired with easy-to-handle anions. Laboratories in Europe and Asia took the lead, building on the foundation laid by research into imidazolium salts. Each new cation-anion combo created a new tool for organic synthesis, extraction, or materials science. Researchers discovered that modifying side chains on the imidazolium ring, such as by attaching an ethoxyethyl group, brought new physical traits and chemical properties. Over the last two decades, teams around the world fine-tuned methods for making these ionic liquids cleaner, more affordable, and better tailored for chemical engineering or pharmaceutical applications.
Product Overview
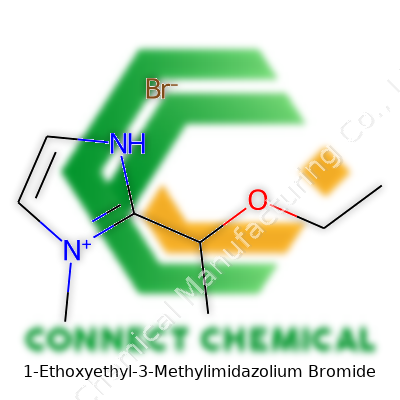
1-ethoxyethyl-3-methylimidazolium bromide stands out as a member of the imidazolium-based ionic liquids family. Its structure features a five-membered imidazole ring, with methyl and ethoxyethyl groups attached to the nitrogen atoms, giving it a unique shape and functional profile. The pairing with bromide brings workable solubility and stability, letting it slot into many roles across laboratory and industrial scales. As a product, it arrives most often as a pale, viscous liquid, sold in glass or plastic containers with tamper-proof sealants to prevent contamination or breakdown from moisture. Chemical suppliers emphasize its purity and residual water content, usually offering analytical certificates and detailed spectra to assure labs of product integrity. Labels include critical details like manufacturer, batch number, purity level, and recommended shelf life.
Physical & Chemical Properties
A big draw for 1-ethoxyethyl-3-methylimidazolium bromide is its liquid state at room temperature. Densities hover around 1.25 grams per cubic centimeter. As with many ionic liquids, its negligible vapor pressure helps limit evaporation losses and fire risks. High polarity, paired with a capacity to dissolve both organic and inorganic compounds—especially those that won’t dissolve in water—make this salt a go-to solvent for tough chemistry problems. Its thermal stability extends up to about 250°C, allowing use in high-temperature applications without risk of decomposition. The liquid’s high viscosity sometimes slows mixing, but thoughtful lab setup usually addresses that. Its conductivity runs in the middle for ionic liquids, good enough for electrochemical cells but not on par with specialized salts for batteries.
Technical Specifications & Labeling
Product requests start by listing minimum purity levels; 99% or higher marks are standard for research-grade. Water content under 500 parts per million keeps hydrolysis and unwanted side reactions in check. Analytical standards require reporting residual organic solvents—often below 0.2%—and clear labeling of any detected halide impurities. Labels include UN numbers for transport, hazard pictograms for compliance with global shipping laws, and batch traceability for recall safety or process improvement. Safety data sheets spell out storage conditions, usually cool and dry, under inert gas if kept long term. These details shield against unwanted chemical changes and keep users safe.
Preparation Method
Chemistry teams synthesize 1-ethoxyethyl-3-methylimidazolium bromide by first alkylating methylimidazole with 1-ethoxyethyl bromide. The reaction, typically run in a dry polar solvent, requires slow addition of the alkyl bromide to prevent runaway heat. Engineers use inert atmosphere—nitrogen or argon—and temperatures below 60°C to reduce side product formation. After stirring for many hours, the reaction mixture gets washed and purified, most often through repeated extraction or vacuum distillation. Residual solvent and excess reagents get stripped out, and the remaining oil is dried under reduced pressure over molecular sieves. Quality assurance teams measure impurities, ensuring every batch holds up under strict analytical standards.
Chemical Reactions & Modifications
Researchers in synthesis labs bend the flexibility of this bromide salt to their advantage. Its compatibility with base-catalyzed and acid-catalyzed reactions, and ability to host transition metal catalysts without corrosion or inactivation, make it popular in cross-coupling or cycloaddition chemistry. Chemical teams find that swapping out the bromide ion for others like BF4- or PF6- leads to unique solubility and reactivity patterns, which helps them fine-tune reaction outcomes. Further tweaks to the ethoxyethyl or methyl substituents change its electrochemical window or viscosity. Chemical engineers keep exploring new uses, from catalysis to separations, often guided by firsthand experiences from competitors who are quick to publish or patent any successful twists.
Synonyms & Product Names
Chemical suppliers and researchers often use shorthand for 1-ethoxyethyl-3-methylimidazolium bromide, including terms like [EeMIM][Br] or EE-MIM Br. Literature from Asia sometimes drops the “1” in the ethoxyethyl identifier, but the backbone remains clear. Some databases record the salt as 1-(1-ethoxyethyl)-3-methylimidazolium bromide, which means databases need cross-referencing to ensure researchers work with the right compound. Other times, catalogues use proprietary product codes, but the imidazolium core always links back to the original chemical.
Safety & Operational Standards
Workplace safety teams stress the handling of ionic liquids with gloves, goggles, and splash-proof coats, since some users report skin irritation or mild allergic reactions. Chemical safety officers insist on chemical fume hoods because splashing or vapor contact could cause health issues. While ionic liquids like this one tend to offer less risk of fire or explosion compared to volatile organic solvents, good practice means never leaving them open to the air for long, to limit contamination or unexpected reactions. Disposal plans should follow local hazardous waste guidelines, avoiding drains or regular trash. Many universities and research labs require annual refresher training on these standards.
Application Area
Labs testing green synthesis strategies appreciate 1-ethoxyethyl-3-methylimidazolium bromide for swapping out traditional organic solvents. Its ability to dissolve cellulose and other biopolymers attracts attention from biomass conversion groups. Chemical process teams found that using this liquid cut waste streams and sped up extraction of rare earth metals and pharmaceuticals. Organic electrochemistry teams value its stable window for reactions where traditional solvents degrade. Materials scientists occasionally use it for templating nanoparticle growth, given its resistance to thermal and oxidative decomposition. There’s real-world value in these applications: greener chemistry, safer procedures, and sometimes sharper yields or better reaction selectivity.
Research & Development
Academic groups remain busy studying new ways to tailor the properties of imidazolium bromides. Industrial partners throw money into programs chasing lower production costs or scaled-up synthesis processes that shrink the carbon footprint. Government-backed research looks for combinations with bio-based cations or “deep eutectic solvents” that play well with existing waste streams. There’s often a tight back-and-forth between fundamental studies—mapping properties, uncovering toxicity—and application-driven work designing new processes. Chemists working closely with industry know performance in the lab doesn’t always translate to process scale, so they keep focusing on reliability alongside innovation. Startups in the green chemistry space pay close attention to any regulatory shifts or toxicity findings, which could shift markets overnight.
Toxicity Research
Toxicologists note some ionic liquids, including 1-ethoxyethyl-3-methylimidazolium bromide, resist natural breakdown, which raises caution for water supplies and ecosystems. Cell culture tests point to moderate toxicity toward bacteria and aquatic life at concentrations much higher than would occur with usual lab handling, but persistent use could add up. Regulatory agencies require reporting any hazardous degradation products. Chronic exposure studies in animal models remain ongoing, but so far acute risks mostly involve skin and eye irritation or mild respiratory symptoms. Environmental safety programs push for improved waste capture and treatment facilities in manufacturing, aiming to keep discharge as close to zero as possible.
Future Prospects
Researchers hungry for cleaner, more tunable chemical processes expect to see imidazolium-based liquids gain ground. Efforts to use bio-derived starting materials for the imidazole ring hope to reduce environmental impact. Ongoing work in catalysis, extraction, and electrochemistry keeps opening new markets for this family of chemicals. Advances in recovery and recycling promise to keep prices steady as more companies adopt these liquids for both pilot and industrial-scale operations. Environmental regulations could shift focus onto non-halide analogs or modifications that speed up natural breakdown. As new data stacks up about health and ecological safety, chemists, engineers, and policymakers keep pushing for better answers, safer handling, and smarter uses of these versatile salts.
Why Chemists Care about This Ionic Liquid
Plenty of specialty chemicals gather dust in catalogs, but 1-ethoxyethyl-3-methylimidazolium bromide stands out for practical reasons. It’s a mouthful, but those who’ve worked in research labs know it shapes how reactions unfold. As a room-temperature ionic liquid, it stays liquid well below water’s boiling point, holding stable even as things get heated during experiments. In short, it makes stubborn chemistry behave.
Pushing the Boundaries in Green Chemistry
These days, there’s been a big push to cut out volatile organic solvents. The pressure is on in my own field, both for workplace safety and cutting down emissions. Ionic liquids like this one barely evaporate, and their low volatility means less toxic fume hoods running nonstop. Researchers keep looking for ways to swap out old solvents in catalytic reactions, and this compound solves real headaches by keeping reactions efficient but safer for everyone in the lab.
Biomass Processing Gets a Real Boost
Talk to anyone working in the fast-moving field of renewable materials and you’ll hear about ionic liquids for breaking down cellulose. Some of my colleagues run into bottlenecks when trying to convert plant waste into fuels or sugars. This imidazolium salt, with its strong dissolving power, takes apart tough plant fibers much faster than classic acids or enzymes. Recovery and reuse of the ionic liquid can drop costs long-term, although scale-up still brings its own growing pains.
Electrochemistry Experiments Become Feasible
A big use in my past projects had to do with supporting electrolytes. Most batteries, fuel cells, and electrochemical sensors need a medium for ions to move. Water-based solutions break down under high voltages, and organic ones dry out or catch fire. Systems built with this ionic liquid support stable voltages and a broad electrochemical window. From lab testing, I found it works in electrodeposition and organic synthesis, which lets researchers push new reaction types without the fear of explosion or power loss.
Material Science and Polymer Synthesis
Labs focused on nanomaterials and advanced membranes benefit, too. When developing polymer gels and conductive plastics, adding 1-ethoxyethyl-3-methylimidazolium bromide to mixtures gives plastic-like materials higher flexibility and added electrical flow. In my past collaborations with materials engineers, we used ionic liquids as plasticizers to create custom membranes for gas separation. The resulting polymers lasted longer under stress and didn’t get brittle at low temperatures.
Navigating the Challenges
Nothing comes without a downside. The price tag for specialty ionic liquids stays high and clean-up isn’t always as simple as draining water. Some studies flagged toxicity concerns for aquatic life. Researchers and industry groups keep exploring ways to recycle and re-engineer these salts for minimal environmental impact. If sustainable production lines and easy recycling methods come through, the benefits will go farther.
Looking at What’s Next
From my point of view, cutting-edge labs and startups embrace this compound for dissolving tough feedstocks, supporting safer batteries, and pushing catalysis forward. As chemists keep demanding safer, more reliable solvents and electrolytes, learning from real-world trials with this imidazolium salt will keep shaping the toolkit. The best results come from sharing what works and tweaking the recipe, not just relying on textbooks or company datasheets.
Exploring What Makes This Salt Unique
1-Ethoxyethyl-3-methylimidazolium bromide lies at the intersection of organic chemistry and practical laboratory applications. Chemists often use this ionic liquid to push boundaries in green chemistry and create more sustainable processes. The structure—combining an imidazolium ring with ethoxyethyl and methyl groups—results in a compound that catches both academic and industrial attention.
Breaking Down the Structure
Let’s talk atoms and bonds. The core piece is the imidazolium ring, which has two nitrogen atoms spaced three carbons apart, forming a five-membered aromatic ring. This skeleton favors charge distribution, letting the molecule stay stable yet reactive in the right conditions. On one side of the ring, a methyl group tacks onto the nitrogen, making it more hydrophobic. On the other side, an ethoxyethyl group attaches to the remaining nitrogen, lending flexibility and solubility in organic solvents. Pair this cation with a simple bromide anion, and you have a salt that flows like oil instead of forming crystals.
The structure can be written in line notation as C8H17N2OBr. The imidazolium ring keeps the nitrogen atoms at positions one and three. Add a methyl to one nitrogen and a 1-ethoxyethyl group to the other, and balance it out with a bromide ion. This simple design influences everything from melting point to chemical stability.
Why Scientists Care About This Compound
The arrangement of substituents on the ring can change its interaction with different solutes. In practice, this gives researchers a reason to substitute their go-to organic solvents with ionic liquids like 1-ethoxyethyl-3-methylimidazolium bromide. These liquids conduct electricity, dissolve a variety of compounds, and often show low volatility, which makes them suitable for safer lab work and scalable processes. For green chemistry, avoiding volatile organic compounds cuts down on air pollution and improves lab safety.
During a stint working on difficult separations in a university lab, I ran across this family of imidazolium salts. We aimed to separate polar and nonpolar molecules without resorting to traditional solvents full of environmental baggage. The ionic liquid, with its custom-tailored cation, allowed us to pick solubility and polarity like tuning a radio. We could tweak temperature and add water to encourage selectivity, and when bromide wasn’t doing the trick, we tried different anions. The modular nature of the cation’s chemical structure proved its value time and again.
Challenges and Solutions in Using This Salt
No chemical is perfect, and 1-ethoxyethyl-3-methylimidazolium bromide runs into trouble if handled carelessly. Bromide ions can sometimes catalyze side reactions or corrode equipment. That being said, a little smart engineering around compatible materials and strict procedural controls keeps problems manageable. Regulatory bodies continue to research long-term environmental and health impacts of ionic liquids, and some early data shows promise—though every lab and production plant should keep a close eye on waste handling.
For scientists looking to cut solvent waste, ionic liquids like this imidazolium salt give a lot of hope. The chemical structure isn’t just a picture in a textbook; it sets the rules for how the compound works in everything from simple syntheses to complex industrial separations. By understanding and tweaking these building blocks, chemists pave the way for cleaner, safer, and more efficient chemical work.
Getting to Know the Chemical
1-Ethoxyethyl-3-methylimidazolium bromide pops up in research labs across the world, especially among chemists looking to harness the power of ionic liquids. These liquids promise less volatile alternatives in various reactions, and the imidazolium family sits at the core of this field. People lean on ionic liquids for their stability and reduced emissions compared to common organic solvents. Even with this promise, safety takes the front seat each day work begins.
No Substitute for Knowledge
Every person who sets out to handle a compound like this needs real information, not guesswork. Material Safety Data Sheets (MSDS) spell out hazards, proper storage, and the physical characteristics of each chemical. Relying on memory—or stories from busy coworkers—leads to trouble. Bromide salts and imidazolium compounds often show low volatility, sparing lab workers the clouds of vapors that plague other solvents. But low vapor pressure does not mean complete safety. Skin can still absorb this liquid, and splashes can create real risks for the eyes and upper respiratory system. Some ionic liquids show low toxicity on first glance, but chronic exposure may be a different game. The science community learns more each year about the subtle risks tied to repeated contact.
Protecting Yourself and Your Team
Wearing gloves isn’t simply about routine. Nitrile gloves serve well against most organics and ionic liquids. Thin latex offers too little protection for comfort. Even a small spill runs the risk of skin redness or irritation, which signals more damage than you might think. Careless habits around goggles put your eyes in the line of fire. Standard chemical splash goggles give a basic defense, and anyone skipping that step courts disaster. Lab coats shouldn’t collect stains; they aim to shield your clothes and skin. Labs with proper air flow lower any possible chance of irritation. Recirculation fans and fume hoods matter not just for the harshest chemicals, but also for anything less studied.
Smart Storage and Spills
Leaving an open vial of 1-ethoxyethyl-3-methylimidazolium bromide on a shared bench encourages accidents. Dry, cool cabinets away from light protect both the chemical and anyone nearby. Spills still happen in the best labs. Small ones, cleaned with absorbent pads and plenty of soap, remind everyone to keep surfaces organized and clear. Larger messes demand evacuation and calling in trained help if bromide salts scatter. Each technician should feel comfortable reporting near-misses rather than brushing off mistakes. Hiding spills risks far worse later.
Weighing Risks and Seeking Improvement
Some teams focus on minimizing contact using automated pipetting or glove boxes. Shifting from glassware to plastic lines or using lidded reaction vessels reduces splash potential. Substituting even less toxic ionic liquids, or returning to tried-and-true solvents, sometimes prevents headaches. Honest communication about side effects, even headaches or irritation, keeps teams safer. Everyone gains from a culture that encourages asking questions and reporting problems without fear.
Staying Informed and Building Trust
Trust grows from habits—the right posters on the lab wall, clear training before new experiments, and open discussion after a close call. Each lab worker relies on the experience of the group. The broader chemical community works together to clarify safety, and newer studies constantly nudge procedures in a safer direction. Reading up, reaching out for expert advice, and paying attention to small discomforts keeps everyone healthier, every day.
Pursuing Consistency in Chemical Quality
Researchers and folks working in chemical synthesis often face an uphill battle when trying to source clean, reliable chemicals. 1-Ethoxyethyl-3-methylimidazolium bromide, an ionic liquid often found in synthesis labs and emerging green chemistry setups, raises the same questions as any other specialty compound: How pure is the bottle on the shelf, really?
From what’s available in major supplier catalogs, this compound most commonly arrives at a purity grade hovering around 97% to 99%. Sigma-Aldrich, TCI, and Alfa Aesar seem to echo this with their product sheets, promising the kind of cleanliness needed for demanding organic transformations. Oddly enough, these high marks on spec sheets do not always translate perfectly to the flask or test tube.
Why That 1–3% Matters
Laboratory projects don’t all thrive under the same level of scrutiny. In my own work, running NMR on these ionic liquids often reveals ghost peaks. Even 1% impurity sometimes throws a wrench into critical coupling reactions or throws off a catalyst’s expected behavior. Trace water, unreacted starting materials, or left-behind reagents like imidazole can complicate things. Talking with colleagues, I know I’m not alone. Synthetic workups get longer. Purification steps multiply. Suddenly, the convenience of a “high purity” commercial source turns into extra hours at the bench.
Purity Isn’t Just a Number
This shortfall gives headaches far beyond the personal. A 2018 analysis out of the University of Birmingham highlighted real discrepancies between stated and actual purities in various ionic liquids, including this cation family. Simple halide tests sometimes flag residual sodium or potassium—even though these remain below what’s listed on the COA. A 99% label often skips past the specific contaminants most likely to sabotage a reaction sequence. Without a full impurity profile, folks can’t always plan for workarounds.
Countless research groups have noted that water content—often measured in ppm rather than percentage—plays an outsized role in ionic liquid stability. Commercial sources rarely offer fresh Karl Fischer titration data, so buyers face a guessing game. This stings when the plan involves anything water-sensitive, like battery R&D or air-free catalysis.
Working Toward Better Practices
The story doesn’t stay stuck in skepticism. Any chemist can take steps, frustrating as they might be. Drying under high vacuum helps, and sometimes passing the salt through activated alumina can knock out persistent halides. Asking suppliers for batch-level impurity data, though not always fruitful, sends a message that buyers care. I’ve found that building a relationship with specialty chemical reps often gets honest answers, even if the answer is “We don’t check for that.”
If the goal is reproducible science—and most of us hate rerunning failed syntheses—choosing a supplier with transparent data matters even more than price per gram. Peer-reviewed published data and supplier COAs both have their roles. Balancing skepticism with routine checks (TLC, NMR, Karl Fischer) slowly builds the kind of confidence that raw numbers cannot.
Chasing perfect purity in a quirky salt like 1-ethoxyethyl-3-methylimidazolium bromide never feels finished. But asking tougher questions about what’s in the bottle and sharing honest results keeps the science moving and the frustration to a minimum.
Why Safe Storage Keeps Your Science On Track
I’ve seen plenty of labs risking good chemistry by letting responsible storage slide. 1-Ethoxyethyl-3-Methylimidazolium Bromide—a mouthful for sure—reminds me that even seemingly stable chemicals need a lot more respect than a casual spot on a shelf. It might not explode at a glance, but proper storage keeps both the research results and the people safe. Those rules come from real lessons learned, not just by-the-book protocol.
Humidity: The Sneaky Spoiler
Dampness finds its way into every gap. Ionic liquids, like this one, soak up moisture the same way a towel does. Put the bottle somewhere humid, and the properties start drifting off from the values you rely on. I've opened bottles left carelessly in busy teaching labs—crusted powder, unknown liquids pooling, unpredictable color changes. Reliability is gone; future experiments get risky results. A dry atmosphere, ideally in a desiccator with fresh drying agent, cuts the problem off at the source. Storing in tightly sealed amber glass doesn’t just look official—it blocks both water and unnecessary light from sneaking in and messing with your sample.
Keep the Temperature Steady and Sensible
Room temperature works for most shelf-life needs, as long as you avoid thermal swings. Heat speeds up chemical changes, even slow ones you don’t see coming. Freezing usually isn’t required and can sometimes cause its own headaches by forcing water or impurities out as crystals. I saw a colleague’s project set back weeks after repeated refrigeration cycles turned a solid into a series of unreadable sludge layers. Sticking with a stable, moderate temperature close to 20–25°C keeps this risk low. No need for a fridge unless the supplier specifically warns about temperature sensitivity.
Don’t Ignore Shelf Life—It’s Chemistry’s Expiry Date
Manufacturers test these salts for stability, and two years stands as a safe assumption so long as you store it right. Making the label easy to read and marking the date you opened it takes guessing out later. Past this rough timeline, quality might slip—hygroscopic salts don’t let you know when they’ve grabbed too much water or started to degrade. Weird smells or cloudiness show something’s off, and tossing a suspicious batch saves more time than trying to salvage half-reacted junk.
Practical Solutions for Everyday Lab Life
A designated cabinet for reactive ionic compounds eliminates confusion. Mixing incompatible chemicals in the same place spells trouble; no one benefits from unknown side reactions in your storage area. Training new lab members to understand these quirks matters as much as actual fire extinguisher drills. For regular users, getting some simple stock control into practice—logging each batch, checking for weirdness on opening, and rotating oldest supplies forward—means you’ll rarely face the “do I trust this?” moment when deadlines loom.
Final Thoughts—Why Details Matter
Ignoring the quiet details of storage cuts corners in research. Keeping those bottles dry, cool, and sealed sets up every experiment for fewer surprises and better, more trustworthy data. That lets real science happen—safely, sensibly, and with fewer headaches along the way.