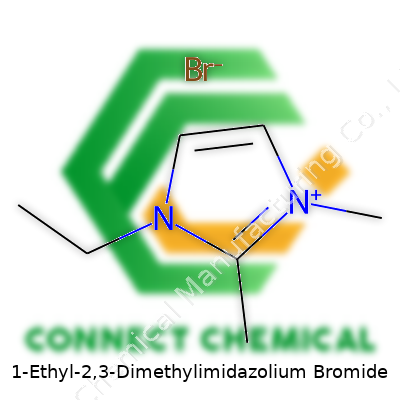
1-Ethyl-2,3-Dimethylimidazolium Bromide: A Practical View
Historical Development
Chemists learn early about imidazolium salts, and 1-Ethyl-2,3-Dimethylimidazolium Bromide (EDMIM Br) has followed an interesting path since its first synthesis. Early ionic liquids like EDMIM Br changed the landscape for lab work aiming for solvents that don't evaporate or catch fire easily. In the 1990s, research groups in Eastern Europe and Japan were already studying the behaviors of imidazolium salts in non-traditional reactions. EDMIM Br emerged as a model compound for those exploring low-temperature molten salts. Journals from the late 20th century started reporting its application as both a reagent and a solvent, underlining the shift from classic organic solvents to more sustainable approaches. This wasn’t just an idle move. Chemists saw new opportunities for reduced volatility, lower toxicity, and the promise of better industrial safety.
Product Overview
EDMIM Br comes as a white to faintly yellow crystalline powder that often attracts moisture if left open. Its structure contains a bulky imidazolium ring sporting ethyl and methyl groups, giving it strong polarity and a unique balance between solubility and stability in water and some organic solvents. Buyers usually find it in high-purity grades for use in advanced synthesis or analytical work. Those who want reliable reagents in bench chemistry often turn to EDMIM Br since it handles a variety of roles, from ionic liquid preparation to phase-transfer catalysis. The product ships in sealed containers, which helps prevent water uptake and ensures reliable results batch after batch.
Physical & Chemical Properties
As a solid at room temperature with a melting point hovering around 130°C, EDMIM Br stands out from more volatile organic solvents. Its thermal stability pushes well above many lab reagents, so it works in devices needing elevated temperatures. In water, it dissolves easily, and in polar solvents like DMSO or DMF, it creates clear solutions. The imidazolium core resists decomposition in air or light, while the ethyl and methyl substituents control its bulk and solubility. The bromide counterion not only ties up reactivity but also allows straightforward exchanges for other anions in custom syntheses.
Technical Specifications & Labeling
Labs rely on the specs: purity above 98%, moisture content less than 0.1%, and bromide content matching the molecular stoichiometry. Labels often carry the molecular formula (C7H13N2Br), structural formula, batch number, manufacture date, recommended storage, and hazard pictograms. Safety data sheets describe routes of exposure, recommended PPE, and what to do in case of spills. Proper labeling pays off: having clear traceability and up-to-date information keeps workflows smooth and standards compliant, especially under regulatory eyes.
Preparation Method
Crafting EDMIM Br looks simple if you have the right skills: start with N-ethyl-2,3-dimethylimidazole as the base, then quaternize with methyl bromide under chilled, controlled conditions. Solvents like acetonitrile or dichloromethane often help dissolve the reactants, and the product precipitates as an off-white solid that gets washed with cold solvents and vacuum dried. Scale-up means keeping a close eye on reaction exotherms and venting any methyl bromide safely, since it's not forgiving to mistakes. Academic labs favor stepwise addition and temperature ramps, letting the reaction finish overnight for maximum yield.
Chemical Reactions & Modifications
Chemists appreciate how EDMIM Br serves as both a starting material and a reaction medium. Swapping out the bromide with other anions broadens its reach, letting researchers synthesize ionic liquids with custom solubility or reactivity. EDMIM Br stands up to mild oxidizers and bases, though strong nucleophiles can cleave the alkyl groups under heat. In cross-coupling reactions, EDMIM Br sometimes replaces traditional organic solvents, offering an environment that supports green chemistry goals. With custom modifications, it's easy to adjust the substituent pattern, opening up a range of analogs for pharmaceutical or materials studies.
Synonyms & Product Names
When looking through catalogs or databases, EDMIM Br wears several hats: it goes by 1-ethyl-2,3-dimethylimidazolium bromide, [EMMIM]Br, and sometimes just dimethyl-ethyl imidazolium bromide. Brand names in research supply chains may append the abbreviation or state purity grades, but the core chemistry stays the same. Anyone buying or selling this compound should cross-check names and CAS numbers to avoid confusion in orders or regulatory filings.
Safety & Operational Standards
Working with EDMIM Br requires gloves, eye protection, and good ventilation, as the compound, like most ionic liquids, displays low but not negligible toxicity. Industrial safety sheets remind chemists to avoid heating it above recommended limits and to avoid direct skin contact. Disposal needs careful neutralization and dilution since bromide ion affects aquatic systems downstream. Laboratories and factories following ISO and local regulations tend to see fewer accidents and less downtime, setting a good example for others growing into chemical handling.
Application Area
EDMIM Br finds strong use in chemical labs focused on ionic liquid chemistry. Battery researchers look for stable, non-volatile electrolytes, and EDMIM Br delivers dependable results in early-stage testing. In extraction chemistry, its ability to dissolve polar and nonpolar chemicals side-by-side has opened up new bioseparations and precious metal recovery schemes. Polymer labs use it as a template or solvent during the synthesis of high-value materials. Some groups have even explored it in soft electronics, where ionic liquids double as both conductive media and flexible supports. Since the early 2000s, demand from green chemistry circles has only pushed its reach further.
Research & Development
The story of EDMIM Br in R&D continues to expand each year, as new uses come to light. Teams working on catalysis see it as a tool to drive reactions that struggled in classic solvents. Process engineers develop continuous flow methods where EDMIM Br not only supports but speeds up conversions. Its use in biomass processing and selective extraction of pharmaceuticals shows a cross-over between traditional organic chemistry and emerging sustainability targets. Many academic groups collaborate with industry now, aiming for new derivatives or composites that can handle higher temperatures, aggressive chemicals, or unusual substrates.
Toxicity Research
Toxicology studies in the past ten years look more seriously at the breakdown products and environmental mobility of EDMIM Br. Compared to traditional solvents, EDMIM Br scores favorably on many acute toxicity measures, though some studies note moderate effects on aquatic life at higher concentrations. Researchers monitor its biodegradability and removal in water treatment processes, finding that the bulky imidazolium ring resists breakdown more than simple amines or alcohols. Routine exposure in properly equipped labs causes few problems, but industrial spills or improper waste management make a strong argument for tighter best practices and research into safer alternatives.
Future Prospects
Future trends suggest EDMIM Br’s use will keep expanding as more industries turn to ionic liquids for greener, safer production methods. The need for solvents that don’t evaporate, don’t ignite, and withstand strong acids or bases keeps driving demand. Improvements in process chemistry might find new ways to recycle or recover EDMIM Br after use, shrinking waste and improving lifecycle performance. Researchers keep pressing for lower-toxicity analogs, degradable by design, hoping to balance utility with reduced long-term ecosystem impact. As regulations on VOCs and hazardous solvents grow tighter around the globe, EDMIM Br stands ready to fill old gaps with new chemistry, providing those in science and industry with a solid, reliable alternative.
Breaking Down Its Name and Value
It’s easy to get distracted by the long chemical names. 1-Ethyl-2,3-Dimethylimidazolium Bromide sounds technical, but it’s actually a special kind of salt known as an ionic liquid. Unlike table salt, this material doesn’t stick to solid or liquid categories. It usually comes in a liquid state at room temperature, which opens up some interesting doors in both research and manufacturing. I watched a friend in the lab work with a vial of it: no fumes, no strong odor, and none of the mess you might expect from classic solvents. It’s not every day that a chemical like this quietly changes the way people solve problems in chemistry and engineering.
Makes Solvents Look Old-Fashioned
Back in college labs, shifting from traditional, smelly organic solvents to something less hazardous always felt like a rite of passage for green chemistry. People have started reaching for ionic liquids such as 1-Ethyl-2,3-Dimethylimidazolium Bromide because they are less volatile and less risky to handle. They don’t evaporate like acetone or toluene, so there’s less worry about breathing in anything dangerous. Just knowing there’s a safer, recyclable option gives hope to anyone who cares about lab health and the planet.
Chemical Reactions Get a Boost
Chemists appreciate tools that speed up or clean up their reactions. This ionic liquid often shows up as a solvent or a catalyst for tough transformations. For example, I read work where these liquids help make carbon-carbon bonds or swap out hydrogen for other groups in molecules. The research points out their ability to dissolve a wide range of materials, which builds bridges between water-soluble and oil-soluble substances. Challenges in pharmaceutical production or fine chemicals sometimes hinge on finding the right medium for a reaction, and this liquid has stepped up in a big way.
Electrochemical Devices Tap Into Its Strength
Solid and liquid electrolytes are the lifeblood of batteries, fuel cells, and capacitors. People look for materials that won’t catch fire or break down under an electric charge, and here, 1-Ethyl-2,3-Dimethylimidazolium Bromide has started to build a reputation. Researchers tested its low flammability and stable behavior at different voltages and temperatures, so batteries and supercapacitors last longer and work more safely. Some studies landed on ionic liquids as the answer to keeping lithium batteries safer, especially as mobile devices crave stronger and steadier power.
Sheds Light on Extraction
Separating metals or organic compounds usually eats up energy and creates waste. Companies and lab technicians now experiment with this ionic liquid to pull out rare earth elements or clean up crude mixtures. The liquid handles both the target molecules and the leftovers more gracefully than many classic solvents. Resource extraction or recycling e-waste could gain efficiency and reduce pollution once these methods scale up beyond test tubes.
What Holds Back Wider Impact
No chemical comes without trade-offs. Some ionic liquids require careful handling because of their possible environmental impact if released carelessly. Researchers weigh this risk against the benefits—especially as the industry searches for ways to recycle and reuse the liquids across batches. More work on toxicity and biodegradability promises safer future applications.
Moving Forward With Common Sense
Switching to materials like 1-Ethyl-2,3-Dimethylimidazolium Bromide tackles both safety and efficiency in labs and industry. Real progress means not just chasing what’s next but also checking whether new tools can be reused and have lower footprints. People in science and industry continue drawing on both long experience and fresh research to strike that balance.
Practical Steps from Personal Experience
Ask anyone who’s spent time in a laboratory or a warehouse: storing chemicals safely takes more than just a label and a locked door. Let’s say you’re dealing with something as common as hydrochloric acid. At first glance, you just want to keep it from spilling and lock it away from curious hands. In practice, there’s much more at play.
The Importance of Storage Conditions
Consider temperature for a moment. Many of us have seen drums or bottles leak simply because someone stacked them in a spot that gets hit by afternoon sun. A rise in temperature can build pressure inside containers, causing cracks or even explosions for more volatile substances. That’s not just a theoretical risk—it’s happened in busy storage rooms where people thought a bit of heat would not matter.
Humidity sneaks up as well. Some chemicals absorb moisture from the air, leading to clumping, dilution, or unwanted reactions. Take sodium hydroxide pellets. Leave them exposed, and they draw moisture from thin air, creating dangerous lye. Keeping containers sealed tight, stored in a dry place, often keeps people safe more than any written warning does.
Material Compatibility
I’ve seen people grab whatever shelf or drum was available to store new shipments, but chemical compatibility isn’t an abstract worry. Hydrofluoric acid, for example, eats through glass but sits quietly in certain plastics. Storing it in the wrong container risks everyone’s safety in the building. It pays off to check the chemical’s data sheet and use containers made from materials the substance can’t degrade. This keeps everyone a step ahead of accidents.
Separation and Organization
Mixing certain chemicals—like acids with bases, or oxidizers with organics—can lead to violent reactions. Some workplaces use color-coded shelves or segmented rooms to emphasize this lesson. I remember a facility that put all flammable liquids far away from oxidizers, each with clear signage and physical barriers. That’s not just smart—it prevents chaos.
Adequate Ventilation
Gases released during storage might not seem like a huge deal until someone opens a cabinet and catches a lungful. Volatile chemicals or those that off-gas need well-ventilated storage, not just any empty closet. Local exhaust ventilation—like a fume hood or cabinet with a vent—can keep air clean and workers healthy. The best labs and warehouses inspect their air flow regularly. You notice the difference right away compared to places that ignore ventilation—smells linger, headaches pop up, people avoid the room.
Clear Labelling and Accountability
In my experience, a simple printed label can be the difference between safety and panic. Marking everything with chemical names, hazards, concentration, and storage date makes inventory checks smoother and helps even new hires make smart choices. Regular training sessions, spill drills, and honest record keeping stop mistakes before they start.
Fact-Based Measures Reduce Risk
Reports from the National Fire Protection Association and OSHA show that most severe chemical accidents start with the wrong storage setup—not dramatic spills during use. Clutter, unsafe stacking, and improper containers lead to leaks and fires. By investing time in good labeling, choosing compatible materials, and separating reactive chemicals, businesses keep employees and the environment safe. Regular inspections and real investment in safety equipment, like spill kits and fire suppression, push that protection further.
Concrete Solutions
Best practices grow from experience. Use secondary containment to catch leaks. Equip rooms with temperature and humidity monitors. Schedule routine checks and make sure everyone knows the basics of chemical safety. These steps turn safety requirements from theory into day-to-day habits that keep everyone out of harm’s way.
Chemicals: Friend and Foe
I remember doing research in my college lab, handling all kinds of chemicals with names longer than the instructions on their labels. Most were harmless with careful use, but every so often, something came along that demanded respect. 1-Ethyl-2,3-Dimethylimidazolium Bromide belongs to a group called ionic liquids, chemicals that draw interest for their role in industry and research. They help with everything from catalyzing reactions to cleaning up heavy metals. Yet, a new material in the lab always leads to the same question: how risky is it to handle?
Information from Research
A look at studies published in reliable journals shows that many imidazolium salts cause trouble for living organisms if exposure gets out of hand. Animal studies from places like the National Institute for Occupational Safety and Health show that similar chemicals slow down cell growth and even hurt organs at higher doses. So, it’s not a stretch to think that 1-Ethyl-2,3-Dimethylimidazolium Bromide can become dangerous if inhaled or swallowed in any real amount, or if it sits on your skin for too long.
Real-World Stories and Regulation
Industrial chemists work with these materials using gloves, eye protection, and fume hoods. Not just because someone said so, but because skin rashes and eye irritation show up too often when safety steps get skipped. European chemicals safety agencies already mark similar materials as irritants. Some lists in the United States mark these chemicals as substances of concern that require detailed documentation, even in small-scale research. Regulatory agencies push for good storage habits and frequent checks since spills or leaks can hang around in the environment, harming aquatic life as well.
What Makes It Different?
What separates 1-Ethyl-2,3-Dimethylimidazolium Bromide from gasoline or bleach is its persistence. Its chemical structure resists breaking down quickly, so a tiny spill doesn’t just disappear overnight. In my old lab, we learned this the hard way after a spill that seemed small at first, but kept showing up in sample contamination for weeks. Used carelessly, even a teaspoon can harm local water systems, going straight through soil into lakes and rivers.
Why More Attention Is Needed
Most folks don’t bump into this chemical at home, but research organizations, universities, and industries use it more with each passing year. I’ve seen overworked lab members cut corners with gloves or ventilation, either to save time or because no one explained the risks clearly. These moments stress me out—no safety measure is too small for a persistent substance. Clear, honest training should become standard, not a suggestion. Companies and academic labs benefit by setting routines that require accountability: written logs, spill drills, and routine air testing, even when no visible vapor escapes.
The Path Forward
Imidazolium salts like this one have value when handled with respect, and lives change for the better if shortcuts disappear from the workplace. Regulators and employers should push for upfront safety data sheets that skip the scientific jargon and give staff real facts. My own time in the lab taught me that the smallest habits—like checking gloves for holes, or speaking up about unexplained symptoms—matter more than expensive monitors. Culture beats equipment every time. Improved awareness, tight routines, and open reporting will protect workers, labs, and the environment from unexpected hazards hiding behind long chemical names.
Why Care About This Chemical?
Working around synthetic chemicals always brings risk, though some folks don’t seem bothered until there's a problem. 1-Ethyl-2,3-Dimethylimidazolium Bromide sounds like a handful, and that’s no exaggeration. This compound crops up in laboratories, battery research, and sometimes in specialized industrial processes because it works as an ionic liquid. Unlike water or oil, ionic liquids are salts that don’t evaporate at room temperature, which makes them handy in the world of chemistry. But convenience in the lab doesn’t mean it plays nice with your skin, lungs, or the environment.
Real Everyday Risks
I’ve handled plenty of bottled chemicals over the years, and a recurring lesson is that just because a substance doesn’t burn your skin on contact or fill the room with fumes, you shouldn’t assume it’s harmless. 1-Ethyl-2,3-Dimethylimidazolium Bromide usually comes as a solid or thick liquid. Once it spills, it sticks around longer than you’d expect, and there’s almost always nobody eager to clean it up. Inhaling its dust or touching it with bare hands invites trouble: skin irritation, eye damage, or worse—long-term health effects that show up down the road.
Some ionic liquids, including this one, linger in wastewater and don’t break down easy. That means they can slide right into lakes and streams if folks pour them down the drain, traveling through systems not built to filter them out. As one study from the Journal of Hazardous Materials found, these compounds may mess with aquatic life and stick around in the environment. We shouldn’t add more to the problem pile.
Solid Handling Tips
Day-to-day, I keep three things in mind every time a bottle of this stuff comes out. Closed shoes, decent gloves, and safety goggles come first. No coat sleeves brushing open containers and no open coffees nearby. A fume hood goes a long way, and I never take shortcuts, even late in the day. After a colleague got a nasty rash once, I learned to double-check gloves for holes before starting.
Label everything. Nothing is worse than finding an unlabeled jar and not knowing if it’s as dangerous as it looks. If I see flakes or powder nearby, I clean it up with damp disposable towels, not a vacuum—the last thing you need is this getting blown into the air. All waste goes to a special bin, not the regular trash.
Responsible Disposal Steps
There’s a reason universities and companies drill this next point into you: chemicals need real disposal plans. I pack solid waste in leakproof containers, clearly marked, and hand it off to my institution’s hazardous waste contractor. Liquid mixes go the same route, sealed and labeled with exact contents. It’s tempting to skip steps, but it only takes one mistake to cause someone a headache, or worse, an environmental crisis.
Tougher rules in places like Europe and California show there’s mounting concern over chemicals like these. Regulators set disposal limits for a reason—some cities can fine labs that dump forbidden chemicals down the sink. Sharing lessons, pushing for clear staff training, and checking inventory so containers don’t linger forgotten in a back cabinet all make a difference. Responsibility starts in the lab, but it matters in wider communities, too.
Possible Solutions for Better Safety
I’ve seen more labs moving to scheduled waste pickups, proper staff training, and switching over to less risky compounds where possible. Sustainable practices aren't just a buzzword. Researchers and managers push for greener alternatives wherever possible, and share disposal instructions with anyone new to the space.
It boils down to this: we’re only as safe as our least careful coworker. Taking shortcuts with disposal costs more in the end than handling chemicals right from the start.
Digging Into Purity
Ask someone in the lab what purity means, and you’ll get practical answers. Purity decides whether your chemical passes quality control or gets tossed in the hazardous waste bin. Researchers count on purity to avoid surprises in their results. Whether it’s a drug candidate or a material for electronics, a single percentage point can spell trouble, so it’s easy to see why questions about purity never grow old.
Purity has always been about more than a single value stamped on a label. For example, if the label promises 99.9% and a batch tests at 97%, there’s a whole process of investigation. Impurities don’t just dilute—they can react, block, or even poison a reaction. In pharmaceuticals, impurities have made headlines for causing patient harm. That’s a personal reminder: purity checks save lives. In other fields, low-purity chemicals sabotage electronics or soil new polymers. No shortcut here.
People working in regulated labs use high-performance liquid chromatography (HPLC), gas chromatography (GC), and spectroscopic techniques to judge purity. Results must be repeatable and traceable—labs that skip this step risk embarrassment or worse if something goes wrong. More than once, I’ve seen entire shipments recalled, and it always comes back to missed steps in routine purity testing. The point is, purity isn’t just a box to check. It’s a sign of trust. If you can’t trust a chemical’s analysis, the whole workflow gets shaky.
Molecular Weight at the Core of Function
Molecular weight might seem like a dry number picked from a table. In reality, it tells you how much material you’re holding and directly impacts everything from dosing to solution prep. If I’m scaling up a reaction, accuracy matters—misjudging molecular weight leads to ruined batches, wasted money, and lost time. In polymer science, molecular weight isn't just a single value but a distribution, which ends up shaping the physical properties of a final product.
In drug development, molecular weight affects absorption, distribution, and elimination. A molecule too large may never reach its target in the body, or it may linger too long. Some drugs are abandoned simply because the molecular weight places them outside the “drug-like” window. This isn’t academic; it shapes how treatments get designed, tested, and brought to market. Hard lessons were learned when results from rodent studies failed to translate to humans due to molecular weight discrepancies.
Putting Knowledge Into Action
It’s tempting to cut corners and take labels at face value, especially in a busy lab. My own work has forced me to double-check every claim, no matter how reputable the supplier appears. I’ve seen peers run into costly snags: an experiment veering off course because a solvent contained hidden contaminants or a synthesis stalling after relying on outdated molecular weight data.
Problems like these can feel overwhelming, but there’s a straightforward fix. Labs should keep up-to-date certificates of analysis (COAs) for every critical reagent, and analysts should spot-check each new batch—especially when dealing with sensitive or high-value projects. Don’t delegate this to just one person; build a culture where everyone feels responsible for what goes into the workflow.
Chemicals with known purity and molecular weight reduce risk and support solid science. If research aims to solve real problems, then these checks are as necessary as any fancy instrument or innovative technique. Ask questions, document results, and never take “good enough” for granted.